
Abstract
The medical device and pharmaceutical industries play an essential role in the development of cardiovascular devices and drugs, and industry employees are frequently listed as co-authors of clinical trials published in peer-reviewed journals. Potential conflicts of interest in biomedical research have attracted significant attention in recent years, but issues and challenges surrounding authors who are industry employees have not received nearly as much scrutiny. We present a comprehensive discussion of the concerns and challenges regarding the role of industry in the authorship of scientific manuscripts. Academic co-authors, industry employees, the editors of medical journals, and, most importantly, readers, need to consider the perception and implications that accompany industry employee authorship. Potential concerns include the effect of industry authors (and industry support) on study design, data analysis, interpretations, conclusions, and, ultimately, scientific content. Meaningful contributions from industry employees must be acknowledged and reported in scientific and clinical publications. Efforts to provide full transparency on industry support and the role of industry contributors are necessary to maintain confidence in the reports of studies with industry involvement.
Abbreviations
ARO: academic research organisations
CRO: contract research organisations
FDA: Food and Drug Administration
ICMJE: International Committee of Medical Journal Editors
Introduction
Industry-sponsored research is essential to the development, regulatory approval, commercialisation, and subsequent clinical application of new medical devices and pharmaceutical agents. Industry employees who participate in this research may come from varied clinical and scientific backgrounds, and include physicians, basic scientists, nurses, epidemiologists, health economists, engineers, statisticians, and pharmacologists. These industry employees can have robust academic pedigrees and convey considerable expertise to basic and clinical investigations; thus, synergistic research collaborations between academia and industry can benefit both sectors. Furthermore, given the enormous costs associated with medical research, the academic medical community often relies on collaborations with (and support from) industry to answer important clinical questions1,2.
Despite the positive contributions of these academic-industry collaborations, issues relating to conflicts of interest in biomedical research have also attracted significant attention over the past decade3-5. One quarter of the investigators engaged in biomedical research and approximately 50% of authors of cardiovascular publications have industry affiliations6. More than half of the experts who write cardiovascular clinical practice guidelines report potential conflicts of interest with industry7. Importantly, industry sponsorship of clinical research has been associated with outcomes and conclusions that are favourable to industry3,8. Cardiovascular disease is no exception, and trials funded by industry and for-profit organisations are more likely to report positive findings for investigational drugs and devices than trials funded by not-for-profit groups9. There are many reasons which may potentially underlie this association; nevertheless, it may contribute to the observation that reporting industry funding in a manuscript negatively influences readers’ perceptions of methodologic quality, and significantly reduces the willingness of physicians to believe the study results10.
Today, an increasing number of industry employees are participating as co-authors of articles published in peer-reviewed academic medical journals, and this has generated a considerable amount of vigorous debate11. Among the discussion points, authors affiliated with or employed by industry may have inherent potential conflicts of interest relating to the publication of favourable study outcomes. Given the ubiquity of industry relationships and the frequency of industry-authored studies, scientific journals have relied on full disclosure to mitigate the impact of potential conflicts of interest12. This approach is not infallible; for instance, among coronary stent trials, significant inconsistencies in author disclosures have been reported13.
Even though industry affiliations may be perceived to jeopardise scientific impartiality in some cases, bias actually arising from these potential conflicts of interest has been difficult to quantify. In one recent analysis, self-declared financial conflicts of interest, including industry employment, did not appear to impact on the results of major cardiovascular trials6. However, this analysis included older publications from only three high-impact journals, and the study included few (12%) device trials. In a more recent analysis of 357 manuscripts of interventional cardiology device trials, 21.8% listed industry employees as co-authors. Though the frequency of positive reported outcomes among manuscripts with and without industry employees was similar overall, among randomised controlled trials, industry employee co-authors were more often associated with positive study outcomes than manuscripts without industry authors (88% vs. 59%, p=0.0008)14. However, in many cases, differences in trial outcomes may simply reflect the immense financial support industry is able to provide to conduct adequately powered, well-conceived, successful clinical trials.
Since potential biases associated with industry authorship are complex, we provide academic and industry perspectives on key issues and concerns surrounding industry employee co-authorship of cardiovascular clinical research. While the discussion presents two perspectives, it is important to recognise that there is not an absolute dichotomy between the two views, and that the views presented do not necessarily reflect those of all members of academia or of industry. Nevertheless, given the increasing opportunity for industry-academic collaborations, and the general recognition that there are potential biases inherent from both the academic and industry sides, it is important to put these issues forward for consideration.
What are the incentives associated with authorship of scientific research?
ACADEMIC PERSPECTIVE
Device and pharmaceutical manufacturers are corporations that typically have obligations to generate income for their shareholders. As such, there may be corporate pressures to demonstrate clinical “success”. Employees of industry are often loyal to their workplace and/or have financial interests at stake, including job security, salary, and company options or stocks. Therefore, industry employees can have incentives to publish data that support the use or enhance the reputation of their device or drug. These pressures may inform study design, study conduct, the reporting of findings, and manuscript authorship. As a consequence, although most industry investigators are honest and disciplined scientists, some in academia view industry participation in clinical research with a degree of scepticism.
Academic research organisations (ARO) (and contract research organisations [CRO]) complicate the discussion of conflicts of interest. AROs collaborate with industry clients to provide guidance on trial design and lead industry-sponsored clinical trials. Well-respected scientists and thought leaders from academia lead AROs and confer legitimacy to the industry studies they manage. However, since industry payments support the operations of these entities, there are clear financial incentives for AROs to design and publish successful trials in order to secure future industry contracts. Consequently, in some cases, AROs (and CROs) are viewed as an “arm” of industry by the independent academic community.
INDUSTRY PERSPECTIVE
Concerns have been raised about potential “incentives” and “bias” of industry authors, but it should also be freely acknowledged that there are inherent “academic” biases that may be more subtle, yet may be no less prevalent among non-industry authors10. An author who has built his or her academic career, reputation, and funding support on a particular tenet will subconsciously (or overtly) tend to position the results of studies in ways favourable to their own views. There may not be direct financial rewards of sales or profitability, but there is the financial impact of future grant support, academic promotion, the likelihood of publishing, the “currency” of positive trials, and leadership in the academic community. Consequently, academic and intellectual conflicts of interest may be every bit as powerful as those associated with industry.
Authorship of academic manuscripts can be important to employees of industry, although often for different reasons than in academia. Whereas in academia there is a Darwinian world of “publish or perish” for advancement and promotion, authorship in industry is viewed as a symbol of high-level involvement and collaboration. Furthermore, in the preclinical realm, authorship provides the opportunity for an industry employee to be identified as a valued academic contributor, acknowledged as an expert by the broader scientific community, and to enhance the employee’s stature within his/her organisation. This is analogous to academia, since many preclinical industry authors are usually accomplished scientists in their own right, and a great deal of work goes on behind the scenes to bring a drug or device forward to clinical application.
In the clinical trial arena there is heightened sensitivity to potential sponsor biases, and most large clinical studies, especially for registration, will have strong clinical and academic leadership. Industry employees (usually within clinical development) may participate as members of the leadership of large clinical trials and may help to facilitate operational aspects of the study.
How does industry sponsorship and funding impact on scientific research and manuscript authorship?
INDUSTRY PERSPECTIVE
In clinical research, “sponsorship” and “support” are two distinct concepts. Sponsorship refers to the party responsible for a clinical trial from the perspective of regulatory authorities. Investigator-sponsored studies are carried out completely under the guidance and direction of the academic investigators. In these circumstances, industry’s role is to determine whether or not to support an investigator-sponsored study based on the proposed design. Industry should not direct or control the design or conduct of the study, the subsequent analysis, or reporting of data. Industry support may come in a number of ways. Support can be provided in the form of contract-driven milestone payments that are fair market value for the activities performed, with mutually agreed upon contracts to define the scope of the work and the anticipated timelines. Support may also take the form of drugs and devices provided free of charge to academic investigators, so that neither patients nor investigators bear this expense. Industry employees generally do not co-author publications reporting results of investigator-sponsored studies. Simply providing funding support is not adequate to qualify for authorship.
In contrast, industry-sponsored studies are not only financially supported by industry but may also have substantial industry input into their design, particularly if the study is designed to generate data for a regulatory submission. Academic physicians may lead the clinical trial, present the data, and co-author publications, but industry scientists may play an equally important role in the design, analysis, and interpretation of the data. As long as they meet accepted criteria for appropriate authorship, industry authors can, and should, where appropriate, be included as authors of industry-sponsored studies.
In recent years, a hybrid category of “collaborative” studies has emerged. These generally start as investigator-initiated studies that are industry-funded, but involve special circumstances such that the academic investigators request additional scientific, analytic, or logistical support from industry. In these unusual circumstances, if an industry employee provides significant scientific support and direction for a study, and meets all the other International Committee of Medical Journal Editors (ICMJE) criteria, authorship should be considered.
ACADEMIC PERSPECTIVE
Financial support of scientific research can give industry significant influence over investigator-initiated study design, even when industry employees do not meet authorship criteria and are not listed as co-authors of the published manuscript. For example, the decision to provide, or withdraw, financial support for investigator-initiated research can lead to premature changes in study design or study termination. Distinctions between sponsorship and support are not systematically reported and may be obscured in the current approach to published disclosures.
How does industry involvement potentially impact on study design and interpretation?
ACADEMIC PERSPECTIVE
When collaborating with industry, academic researchers frequently see themselves as gatekeepers to maintain scientific conduct, focus on the hypothesis, ensure appropriate methods and statistics, and to draw measured and balanced conclusions from the results. Studies investigating the use of novel drugs and devices should be designed and conducted according to rigorous scientific rules and ethics. The design of every study should have scientific merit, even if planned only for regulatory purposes. Academic investigators who work closely with industry on the design and execution of medical device studies often feel strongly that regulatory studies should also be used as a platform to examine additional clinical questions.
Academic investigators frequently criticise elements of industry-sponsored study design. Industry trials often avoid head-to-head drug or device comparisons, unless mandated by regulatory authorities. Selection of a non-inferiority study design is often favoured by industry to report equivalence to established therapies for regulatory approval or to ensure clinical utilisation. However, pre-specified non-inferiority margins vary widely and can include point estimates suggestive of potential significant hazard.
Selection of study endpoints is important. In the modern era, industry-sponsored trials (including those led by AROs) often eschew hard clinical outcomes in favour of surrogate or complex composite endpoints, which may increase the likelihood of a positive trial outcome, but may decrease the clinical applicability of the study. Although there are plenty of industry-sponsored studies that are well-designed trials with appropriate endpoints, the selection of endpoints by industry should be carefully assessed by all investigators, scientific advisory committees, reviewers, and readers alike. When feasible, the rationale for the selection of surrogate outcomes should be explicitly reported in the published manuscript.
With any collaboration between academia and industry, there is the potential for authors from academia to have a different interpretation of the data as compared to the industry-employed co-authors. In addition, decisions may be dependent on legal obligations and/or contractual arrangements between AROs and industry. However, the final interpretation of data and conclusions of the research should be the ultimate responsibility of all co-authors from both academia and industry, in response to public perceptions of academic authors as unbiased members of the scientific community and providing “balance” to industry perspectives.
INDUSTRY PERSPECTIVE
There is wholehearted agreement from industry that studies of novel drugs and devices need to be ethically designed, conducted and reported. However, as is evident from some of the academic views expressed, there is considerable suspicion of industry studies by the academic community. The assertion that the academic world must act to balance the “evil” influences of industry on scientific integrity creates a false dichotomy. Industry-sponsored studies are often designed for regulatory submission and must meet regulatory requirements that can vastly complicate the scope of the data to be collected. Generally, the study design for registration studies originates from the industry sponsor, and subsequently is vetted by an academic scientific committee or governing body to ensure academic rigour, clinical feasibility, and ethical appropriateness. Regulatory agencies also have a major impact on the design of these studies, as industry is mandated to follow regulatory guidance in order for drugs or devices to be approved for clinical use. Rather than including “soft” endpoints, industry-sponsored studies are skewed to the “hard” endpoints that regulators mandate for approval. Furthermore, given the increasing costs of large-scale registration studies, there is considerable momentum to design more efficient, pragmatic clinical trials, with limited data collection directed at focused scientific questions.
The selection and prioritisation of endpoints is of particular interest to industry sponsors when hierarchical analyses are being performed. This is most evident as secondary endpoints are identified for registration studies, given the important distinction between secondary hypothesis-generating subgroup analyses and the rigorous statistical superiority of specific outcome measures. Moreover, when formal non-inferiority analyses are performed, there are well-recognised regulatory standards that may need to be met for non-inferiority claims. Considerable cross-functional work goes into the statistical analysis plans for industry-sponsored studies intended for regulatory approval.
When is industry employee authorship appropriate and how should it be disclosed?
ACADEMIC PERSPECTIVE
Transparency is an important aspect of scientific and medical publications and all authors must report relationships with industry. When industry employees are involved in the design, execution, and analysis of a study, failure to disclose their participation may be misleading and inappropriate. All contributions that merit authorship need to be acknowledged transparently to the scientific and clinical community.
Unfortunately, the role of industry employees in the research process is often not clear to readers of studies published in scientific journals. Many industry authors appropriately contribute to the study design, organisation, and conduct, and rightfully deserve credit. However, in some cases, industry employees (and academic authors) are “honorary” authors who have not contributed substantially to the specific study design, data acquisition, interpretation, drafting of the manuscript, or critical content revision and do not meet ICMJE authorship criteria15. Honorary authorship varies widely based on disclosure requirements, but in older literature has been reported to occur in 4-60% of manuscripts published in three separate high-impact journals in 200216. Honorary authorship should not be part of any credible scientific endeavour. Similarly, authorship must never be bestowed as a form of compensation, or reciprocity for the provision of funding. Chief executive officers, chief medical officers, and other industry leaders involved in product development should not serve as manuscript co-authors unless they have personally made direct contributions to the publication in question. Conversely, industry employees may have provided important input that is not adequately communicated in study disclosures. While this is technically not “ghost” authorship, which refers to uncredited drafting of the manuscript, it is important that industry involvement in study design be acknowledged and appropriately communicated. Unfortunately, due to limitations of disclosure reporting, metrics are not available to determine the extent of each author’s involvement in the research process. When medical writers are contracted to draft the text of a manuscript but do not otherwise meet authorship criteria, their employment and contribution should be stated in published disclosures.
INDUSTRY PERSPECTIVE
Guidelines for authorship have evolved considerably in recent years. The aforementioned ICMJE recommendations are now embedded in the policies and procedures of virtually all major industry sponsors, and mandate all four criteria for authorship15:
substantial contributions to the conception or design of the work; or the acquisition, analysis or interpretation of data for the work;
drafting the work or revising it critically for important intellectual content;
final approval of the version to be published;
agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
In both academia and industry, authorship signifies a substantial contribution to conception or design of the publication or analysis or interpretation of study data, substantial participation in the manuscript, final approval of what is published/presented, AND acknowledged accountability.
The third iteration of Good Publication Practice for Communicating Company-Sponsored Research (GPP3) also provides recommendations for industry authorship policies17-19. This guideline arose as an initiative from the International Society of Medical Publication Professionals, and is intended to provide industry sponsors with ethical guidance on industry authorship, although the principles espoused can be extended to all authors involved in publications. In particular, GPP3 emphasises the publication planning process for industry sponsors, to ensure that there is no commercial involvement and that the highest ethical standards are maintained. In contrast to ICMJE criteria for authorship, in which all authors who meet the first criterion should be given the opportunity to meet other authorship criteria, GPP3 guidelines recommend that “priority should be given to the key contributors who have the necessary background to analyse or interpret the findings”.
“Honorary” authorship is an unfortunate reality among modern-day academics and should be as actively discouraged in industry as it is in academia. In the same way that CEOs, CMOs, and other industry leaders should not serve as co-authors unless they have contributed to a publication, leaders in academia who have not directly contributed to the manuscript should not be listed as authors. The issue of “ghost” authorship by unacknowledged contributors is much more nuanced. GPP3 recommendations substantially restrict who should be included as an author, and they strongly suggest 10 or fewer authors per manuscript. However, a large number of industry employees frequently provide input into study design, data analysis, and interpretation, especially in large multicentre registration studies. There may be complex mechanisms within any given study surrounding the selection of individuals invited to participate in the authorship process once the trial has been designed and executed, and the data have been analysed and interpreted. In reality, this is the point at which the contributors are distinguished from the authors, since in order to fulfil Criteria 2-4 of the ICMJE criteria an author has to participate in the writing process. There may be any number of uncredited individuals who have contributed to study execution and/or analysis, but do not meet full authorship criteria. While it is common to see exhaustive lists of participating investigators (and, more rarely, study coordinators) listed in the appendices of large clinical trials, the contributions from industry are often much more anonymous. A comparison of study protocols and results of industry-initiated trials identified that 75% of final manuscripts omitted individuals who made “significant” contributions (defined as individuals who wrote the trial protocol, participated in the statistical analysis, or helped to write the manuscript) that fell short of ICMJE criteria20. In many cases, these uncredited individuals were industry-employed biostatisticians. Thus, manuscript authorship may represent merely the tip of the iceberg regarding acknowledgement of contributions by industry employees to clinical studies published in peer-reviewed academic journals. However, given the complexity of modern-day clinical trials, full acknowledgement of all “significant” contributors can be challenging.
Concerns have been raised that “positive” studies with industry authors are more likely to be published than “negative” ones. However, authorship in industry is not generally “requested”, nor do employees typically have the ability to pick and choose between authorship of “positive” and “negative” studies. Since industry employees would not author studies that are not industry supported, in essence this becomes a chicken or egg conundrum: do industry authors drive the results, or do “positive” results arise as a consequence of industry involvement (as evidenced by authorship)? Associations between industry involvement and study outcomes are plausible for a number of reasons: 1) industry-supported studies typically have more resources available to ensure successful execution; 2) industry may be less likely to invest in studies anticipated to be neutral or negative; 3) in order to be approved within a given organisation, industry-supported studies must go through multiple layers of rigorous scientific review and “pressure testing”. Thus, the full nature of the association between industry authorship and published findings remains complex and uncertain.
RECOMMENDATIONS FOR APPROPRIATE AND TRANSPARENT INDUSTRY AUTHORSHIP
Employees of drug and device manufacturers frequently author manuscripts published in high-impact peer-reviewed medical journals. Academic co-authors, industry employees, and the editors of medical journals should consider the potential perception and implications of industry employee authorship (and industry support) on study design, data analysis and, ultimately, scientific content. Employment by industry should not preclude authorship of scientific or medical peer-reviewed publications. The more fundamental issue of “appropriate” authorship depends on scientific contributions to the study and adherence to ICMJE criteria for all authors. In fact, because of public sensitivity to the involvement of industry and its employees in medical research, academic and industry perspectives are aligned that full transparency regarding industry contributions and involvement is essential (Table 1, Figure 1).
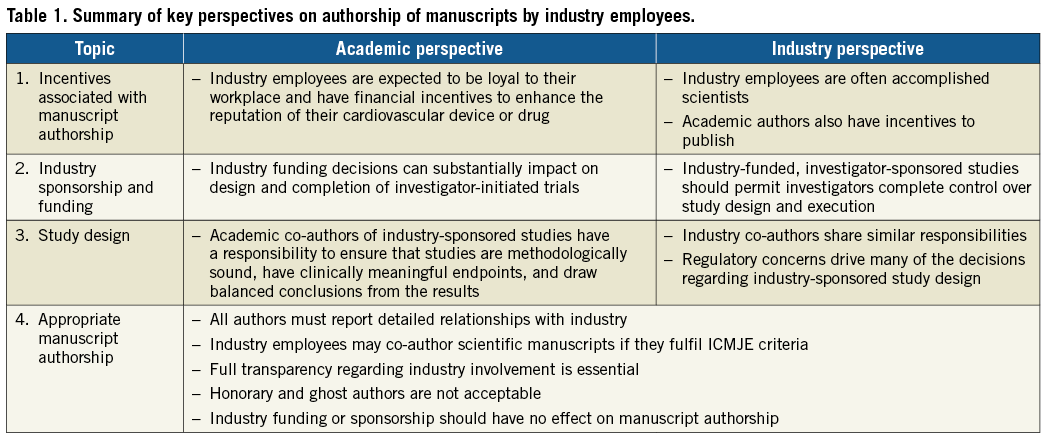
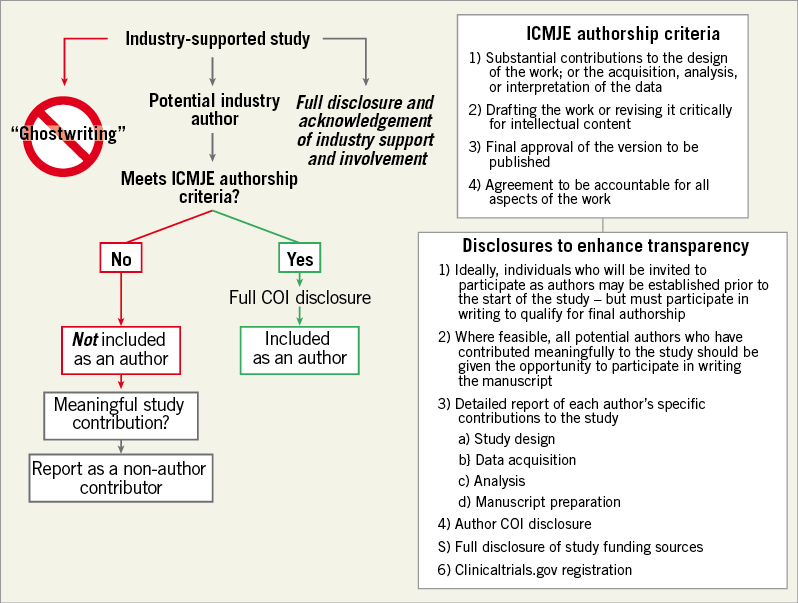
Figure 1. Decision tree to determine eligibility for industry authorship and proposed mechanisms to enhance transparency.
Widespread adoption of comprehensive conflict of interest disclosure reporting and strict authorship definitions based on the ICMJE recommendations may mitigate the potential for perceptions of industry bias in peer-reviewed academic medical literature15,21. Similar to authors in academia, authors employed by industry must meet all four of the ICMJE criteria. The Good Publication Practice (GPP) guidelines for Communicating Company-Sponsored Medical Research also provide a framework for appropriate industry authorship17-19. We recommend that criteria for industry authorship follow ICMJE and the GPP guideline recommendations, and advocate that the reporting of specific contributions of each industry author should routinely be included in all articles (Figure 1). A clear, written acknowledgement of the role that industry played in supporting and executing the study should also be provided. The involvement of all authors in the acquisition, statistical analysis, or interpretation of the data should be clearly stated. When feasible, the data analyses and interpretations that underlie the published manuscript of an industry-sponsored study should be performed in collaboration with (or exclusively by) academic collaborators. If an industry employee is listed as the first or senior author of a manuscript, the specific reason for this unusual authorship position should be reported. Ideally, individuals invited to participate as authors will be designated prior to the start of clinical investigation and documented in the study protocol (although this is not always feasible), and all potential authors who have contributed meaningfully to the study should be given the opportunity to participate in writing the manuscript. This strategy can facilitate adherence to ICMJE and GPP authorship guidelines and ensure appropriate transparency.
The number of authors listed for each manuscript is often long and is sometimes limited by journals. Consequently, there may be limited room to acknowledge all the individuals who have contributed to the study execution and its subsequent publication. An online supplement is a feasible and inexpensive tool to provide a complete list of individuals who contributed to the study, with their affiliations and conflict of interest disclosures. This concept of “contributorship” has been proposed as a solution to ensure transparency and accurately report involvement of all individuals who have made substantial intellectual contributions to a study, even if they do not all meet ICMJE criteria for authorship, and are not listed as authors18,22.
Conclusion
In conclusion, meaningful contributions from industry contributors should always be reported and acknowledged in scientific publications. Efforts to provide greater transparency are important to maintain confidence in studies that have industry support and involvement23-26.
| Impact on daily practice Industry authorship of clinical research has the potential to raise concerns about conflicts of interest. The effect of industry authors (and industry support) on study design, data analysis, interpretations, conclusions, and scientific content should be considered. Full transparency on the specifics of industry involvement is necessary to maintain confidence in studies with industry involvement. Readers should recognise the potential implications that accompany industry employee authorship of scientific manuscripts. |
Conflict of interest statement
G. Weisz sits on the medical advisory boards of Angioslide, Corindus, Medivisor, M.I. Medical Incentive Ltd, TriSol and Vectorious. He is a co-owner/founder of Filterlex. J. Ferguson is a full-time employee of Amgen. The other author has no conflicts of interest to declare.
The views expressed in this manuscript reflect the opinions of the individual authors. Industry played no role in the concept, development, drafting, conclusions or support of this manuscript.

