Abstract
Introduction: Percutaneous Coronary Interventions (PCI) is conducted manually with the operator standing at the bed side, exposed to continuous X-ray radiation. A system where the operator can remotely navigate the wire and device during PCI may improve operator safety and convenience and can possibly enhance procedural precision.
Aim: To develop a remote navigation system (RNS) that will allow computer controlled, remote manipulation of percutaneous coronary interventions..
Methods and results: The remote navigation system (RNS) is designed to handle both coronary guide wire and balloon / stent delivery system and can be loaded either with the coronary wire or with both wire and device in parallel. The RNS is comprised of a bedside unit and a remote manipulation unit. The bed side unit has individual wire and device manipulators, capable of precise maneuvering and positioning of the wires and devices. The system was tested in a wire glass model and was evaluated in a normal coronary sheep model. The wire glass model experiments showed that the wire could be navigated to the required branch and the stent/ balloon adequately positioned, as required. The animal experiments showed that the wire could access any required coronary branch and the stent could be adequately positioned under x-ray fluoroscopy, without causing dissection or other vessel trauma.
Conclusion: A system that enables remote control percutaneous coronary procedures was developed and tested in vitro and in vivo models. Preclinical studies have demonstrated the feasibility of the concept of remote control PCI and have provided the basis for the pilot clinical study of remote control, stent-assisted PCI.
Introduction
Percutaneous coronary interventions (PCI) have become the major method of revascularization for coronary artery disease, with over 2 million coronary interventions performed annually. Stents, including drug eluting stents (DES), comprise the vast majority of current coronary interventions1-3.
In view of the above, stent implantation techniques have matured and standard implantation methods have been widely established. The classical technique which is true for all types of stents involves the use of a coronary guide-wire, manipulated across the stenosis under fluoroscopic control, followed by balloon dilatation, stent implantation and possible additional post-stent dilatation. With DESs, technical aspects of stent implantation that avoid traumatizing non-stented segments may be extremely important in preventing long-term restenosis4. In addition, selections of precise stent lengths to cover the lesion and the entire pre-dilated segment are important technical aspects of the procedure.
Coronary interventions are being performed today with the physician working in an X-ray environment, subjected to a continuous radiation throughout life. This has been unchanged since the field of interventional cardiology began. In spite of protection and the use of lead shields and aprons, the operators are still exposed to non-negligible radiation, with subsequent long-term radiation hazards5-10. Beyond other radiation effects such as the development of cataracts and thyroid disease, the additional risk of fatal cancer due to radiation exposure is increased in proportion to the total cumulative radiation absorption.
Spine problems have also been recognized as a major burden on catheterization laboratory operators. “Interventionalist’s disc disease” is a recognized entity, with cardiologists reporting more neck and back pain, more subsequent time lost from work, and a high incidence of cervical disc herniations, as well as multiple level disc disease11. Wearing lead aprons lead to long term problems, as shown by Kumar et al.12, with 83% of backache problems, neck and shoulder pain. There is little doubt that additional stress on the operator can theoretically lead to degraded performance.
In view of the above, there is growing concern among operators regarding the radiation dose delivered during interventional procedures as well as spine problems that may develop over the years. Despite this concern, no alternative to the current methodology during PCI, where the operator is working at immediate proximity to the x-ray source, has been developed. Navigation in the catheterization laboratory for electrophysiology catheters and for coronary wires have been previously described using magnetic stereotactic techniques13,14. However, the Stereotaxis system does not handle coronary devices in parallel to the magnetic wire navigation.
We therefore developed a remote navigation system for PCI that manipulates the guide-wire and angioplasty devices in a convenient, radiation-free, environment. The pilot clinical study that was recently published15 have provided safety data in patients for simple coronary lesions. The current paper focuses on the design of the system and the preclinical experiments that were conducted in order to show the initial feasibility of the RNS.
Methods: device concept and design
A schematic presentation of the Remote Navigation System (RNS) System® is shown in Figure 1.
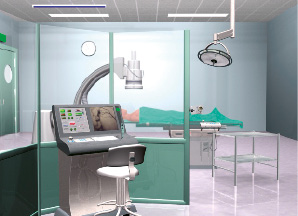
Figure 1. A prospective overview of the cath lab set-up for the remote navigation system. A bed side unit attached to the patient bed is controlled by the console in an X-ray protected environment, either within the catheterization room or at another location. All the procedural operations can be controlled from the console, including wire and device manipulations, balloon inflations, coronary injections and table and C-arm orders.
The bed-side unit is located at the patients table and manipulates the guide wires and balloon/device in a coordinated fashion. The operator control unit which is positioned conveniently, either within the catheterization laboratory behind lead shields, or at the control room, is connected with the imaging systems, as well as the bedside unit and controls the operations through a joy-stick and a touch screen panel.
A view of the prototype bed-side unit, hooked to the catheterization table is shown in Figure 2.
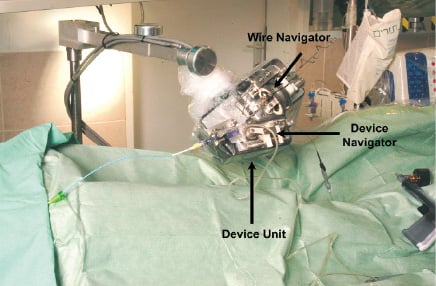
Figure 2. The bed-side unit: the wire navigator controls wire movements. The device navigator advances and retracts the angioplasty device in the axial direction by a set of rollers. The guiding catheter is attached through standard Y-connector holder.
The wire navigator is a detachable unit which navigates the guide-wire by 2 degrees of freedom. The device navigator, which attaches to the device unit, advances and retracts the angioplasty device in the axial direction by a set of rollers. The guiding catheter is attached through a standard Y-connector holder interface to the wire and stent manipulators.
The operator control unit is located away from the patient bed. The set up during the preliminary experiments is shown in Figure 3.
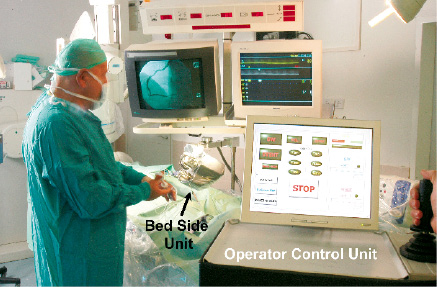
Figure 3. The set-up of the prototype system in the catheterization laboratory. The console was operated from inside the catheterization room. The bed-side unit was attached to the catheterization bed by an adjustable arm. An operator was present at the table for contrast injections and balloon dilatations.
The Operator control unit is comprised of a computer, a touch screen panel to interact with the operator and a joystick for controlling both wire and device.
Modes of operations: The wire is maneuvered using the joystick in a continuous mode, where the wire velocity and rotations are simultaneously operated by the joystick. Any combination between axial and rotational wire motions can be achieved by the joystick position. Discrete wire rotations at 30 degrees angles is achieved using the touch screen mode. It is important to coordinate the wire and device motions, including necessary safety mechanism to prevent accidental dysfunction. The wire can be either loaded alone and driven to its position, or loaded together with the device. In a double loaded mode, when the wire is manipulated the device is locked at its position and vice versa.
The device (Stent, Balloon or other catheter based device) is guided either by a continuous mode, using the joystick, or in discrete steps using the touch screen. The motion is generated by the motored-roller pair, where an additional roller-pair is used to sense the motion of the device. The device is programmed to halt operation of the wire or device if it meets excessive resistance.
Results: preclinical studies
In vitro experiments
The system was tested initially in a glass coronary model with visual feedback of the device movements through the transparent glass. The glass model was used to test the ability to navigate the guide-wire and stent across modeled coronary branches. Multiple passages of the wire and the stent through the different modeled branches were done by several operators. We did not encounter cases in the glass model that the wire / stent could not be driven to its desired location. Similar to real life scenario, occasionally the tip of the wire needed to be reshaped in order to navigate into an acute angel simulated side-branch. In addition to assessing the ability to navigate the wire and stent, the role of the glass model was to provide the basic safety testing for the system, showing proper functioning and proving safety features, such as system halt as it meets resistance and no unintended movements.
These experiments showed the ability of the system to navigate the guide-wire to each branch in the model and to drive the stent over the wire by the operator. In addition, it was used to test the optimal velocities of advance/rotate options of the different actuators. The model has shown that the wire can be easily manipulated to any simulated vascular branch by the advance/rotate modes, and that the device could be easily positioned at the desired location. The system showed the use of the different modes of operation, where continuous fast motion is required to drive the stent close to its final position and then, switching to a discrete mode, the stent is advanced by discrete steps to achieve precise placement.
Animal experiments
The system was then tested in a normal coronary sheep model. The animal experiments aimed to show the ability to safely navigate the guide-wire to the desired branch through coronary intersections and implant a stent with X-ray fluoroscopic control. The animals were premedicated with intravenous Ketamine (10 mg/kg) and Xylasin (2%). Anesthesia was induced by intravenous administration of Pentothal (10-12 mg/kg) and the animals ventilated though an endotracheal tube. Anesthesia was maintained with Isoflurane (1.5%) and oxygen (2-4 L/min) in a closed-circle ventilator system. Intravenous heparin (10,000 units) was administrated routinely. The animal’s arterial blood pressure and ECG were continuously monitored. Following femoral artery canulation with a 7F sheath, a guiding catheter (6-7 F) was used to cannulate the coronary artery. The RNS system was positioned and hooked to the guiding catheter through a standard wire connector and the guide-wire and device loaded. The guide-wire was navigated within the coronary artery and placed in a distal position. Typically the Guidewire could be advanced to its branch or along a vessel using the Joystick in a corkscrew mode, where the wire rotates as it advances. Alternatively, where a specific direction of the tip of the wire was required, either to navigate over a bend or to enter a side-branch the discrete rotation mode followed by an advance “order” was found useful.
Following guide-wire navigation and positioning, the angioplasty device (balloon or stent) was navigated to its position over the guide wire under fluoroscopy control. A proper position within the coronary artery was chosen for balloon inflation/stent delivery and dilatation. Often the device was driven close to its final position using the continuous advance mode. Then, the rest of the motion was conveniently done with the discrete steps mode, taking the stent to its position in 1 or 0.5 mm increments. Following the procedure an angiogram was performed in order to check for possible damage to the coronary arterial tree. The procedure was repeated in each animal several times and eventually, a single stent or more were implanted.
The experiments were conducted in five sheep. Guide-wire manipulations were done with 7 different guide-wires and were successful in all. The guide-wires were successfully positioned at a total of 14 branches. Device (stent/balloons) advancing/positioning was successful in 12 out of 14 attempts. In one case, the stent was not advanced due to RNS malfunction. In another case, the stent delivery system could not be retracted into the guiding catheter by the RNS due to balloon wings causing sliding of the rollers over the device (a safety feature that prevents the use of excessive force). The balloon was manually retracted without any problem or complication. As indicated above, once navigated close to the deployment site with the joystick, final position of the device was done with the discrete steps mode, using the touch screen.
A total of 8 stents were implanted. Positioning was successful in all. There was no evidence of vessel dissection or trauma by angiography. The animal studies have shown that, in a normal coronary model, the guide-wire could be adequately and safely navigated through the natural vessel bifurcations and tortuousity and that balloon angioplasty and stent deployment could be performed using the RNS. It was also shown that both the continuous and discrete steps modes are helpful and complementary for stent advancement and precise positioning.
Discussion
While interventional cardiology has progressed extensively in the development of imaging modalities, therapeutic devices and adjunctive medical therapy, the method of operation, where the physician stands next to the patient table, partially protected from radiation, has remained the same since the days of the first angioplasty procedures three decades ago. We hypothesized that percutaneous procedures can be remotely performed using a robotic navigation system that controls the wires and devices of the angioplasty procedure, as well as a computerized mechanical interface. In order to do this we designed a first prototype that can handle wire and device manipulation and tested it in a pilot clinical study15. The current paper describes the system design and the experimental system that was used to prove the feasibility of the concept of remote control, robotic coronary interventions.
The current RNS is the first clinically oriented system for remote manipulation of PCI. Robotic manipulation of surgical devices has been reported for several applications, including computed tomography-guided biopsy16 and surgery17,18. In fact, it has been shown that such procedures can be done with transatlantic communications19.
Remote manipulations of intravascular catheters have been describes using a magnetically driven system13,14. That system is capable of maneuvering an electrophysiology catheter and has been shown to help navigate magnetic coronary wires. The current system differs from the magnetic system in allowing full navigation of coronary wires and devices in parallel, and in allowing operation in a standard catheterization laboratory using standard wires and devices.
We have shown here using preclinical glass experiments and animal models that PCI with stent implantation is feasible. With the computer controlled mechanical system, the wire can be advanced/rotated and therefore manipulated as needed, and the balloon/stent can be advanced or retracted as required. The unique discrete motion mode was found attractive, allowing precise and accurate positioning. Safety of the RNS was suggested by the ability to perform the entire procedure without coronary damage. Such a safety profile was also shown in the clinical first in human study with the RNS15. It is clear though that more demanding safety information will come from multi-center clinical trials.
The technical problems that caused system halts were acutely dealt by switching to manual operation. Each malfunction was thoroughly studied by the engineers and steps were taken to prevent future malfunctions. None of the system malfunctions (Halts) posed any risk of damage to the arteries, and could be easily handled manually within a few seconds. It is clear that further engineering of the system was required to assure it safety and reliability.
The rationale for a remote navigation system for PCI is both operator safety and procedural precision. Operator safety relates to minimizing operator radiation exposure, and limiting the long-term back and spine consequences of wearing lead aprons5-12. The potential harmful effects of exposure to radiation are well known5-10. As provided in Bashore et al.5 the lifetime additional risk of fatal cancer from radiation exposure in the cardiac catheterization laboratory (0.04% per REM time’s total cumulative REM exposure) can be estimated as 4%. In addition, the effect of back problems on the ability to perform procedures is already becoming a major factor, with operators refraining from catheterization procedures, either temporarily or permanently, due to low-back problems11,12.
Procedural aspects may involve increased standardization and enhanced safety and precision of balloon and stent positioning. Precision advantage has been shown for robotic surgical systems20. While we present data with a remote control system that is fully subjected to operator control, future developments can allow semi-automatic functions for parts of the procedure, and force feedback haptic tools21 that will further enhance the operational aspects of the procedure.
With the current prototype the procedure is based on visual feedback. We believe that most of the control of PCI is visual, and the force felt at the fingers of the operators often reflect friction built up along the catheter, rather than the true force at the contact site. In fact, excessive force by a floppy wire is first detected by buckling of the tip of the wire. Excessive force in the wire and device is first appreciated from the loss of backup. So a good visual feedback gives us substantial information regarding the force that the device encounters at its tip. In spite of that, we do believe that as the field develops, direct force feedback from the appropriate location may add safety and efficiency to the system. We also believe that force feedback may be particularly important for conditions where the amount of force applied by the wire on a lesion may be important, such as during attempts to cross chronic total occlusions with a stiff wire.
The current system is limited to wire and stent navigation. It is obvious that for complete ability to operate the procedure from a remote location the following components need to be integrated into the operator control. 1. An automatic balloon inflation system. 2. An automatic injection system that is integrated with the RNS console. 3. Table and image intensifier operation. 4. Ability to control the guiding catheter for additional support as needed. This would certainly be important if excessive support is needed for difficult cases. Once these features are added, the procedure can be done entirely from a remote location.
In summary, remote navigation system for coronary interventions, including balloon angioplasty and stenting is feasible. Clinical studies will determine the future role of navigation systems for coronary interventions and will expand the horizons of transcatheter therapeutics.

