Abstract
Background: The mean age of transcatheter aortic valve implantation (TAVI) patients is steadily decreasing.
Aims: The aim of the study was to describe the characteristics, the indications for and the outcomes of TAVI in patients <70 years old.
Methods: All patients undergoing TAVI (n=8,626) from the 18 participating centres between January 2007 and June 2020 were stratified by age (70). For patients <70, the indications for TAVI were extracted from Heart Team discussions and the baseline characteristics and mortality were compared between the two groups.
Results: Overall, 640 (7.4%) patients were <70 (9.1% during 2018-2020, p<0.001); the mean age was 65.0±2.3 years. The younger patients were more often male, with bicuspid valves or needing valve-in-valve procedures. They had a higher prevalence of lung disease and diabetes. In 80.7% of cases, the Heart Team estimated an increased surgical risk and TAVI was selected, reflected by an STS score >4% in 20.4%. Five-year mortality was similar (29.4 vs 29.8%, HR 0.95, p=0.432) in the <70 and >70 groups. In the <70 group, mortality was higher for those referred for TAVI due to an increased surgical risk compared to those referred for other reasons (31.6 vs 24.5%, HR 1.23, p=0.021). Mortality was similar regardless of the STS stratum in patients judged by the Heart Team to be at increased surgical risk (32.6 vs 30.4%, HR 0.98, p=0.715).
Conclusions: Use of TAVI in patients <70 is becoming more frequent. The main reason for choosing TAVI is due to an increased surgical risk not adequately represented by the STS score. The outcomes for these patients are similar to those for older TAVI patients. Dedicated trials of TAVI/SAVR in younger patients are needed to guide decisions concerning expansion of TAVI indications. ((ClinicalTrials.gov: NCT04031274).
Introduction
The management of patients suffering from symptomatic severe aortic stenosis (AS) has been revolutionised by the introduction of transcatheter aortic valve implantation (TAVI). Indications for TAVI have expanded from high-risk/inoperable patients, to include elderly patients at intermediate surgical risk as well1. A meta-analysis of all randomised trials comparing TAVI to surgical aortic valve replacement (SAVR) demonstrated that TAVI is at least equivalent to SAVR in terms of two-year mortality across the spectrum of surgical risk2. The frequency of TAVI is growing exponentially and, according to recent data, it is used in 40% of all interventions for severe AS in Europe3. The median age of TAVI patients in current practice is 80 years old (YO)4. Data on the use of TAVI in patients younger than 70 YO are scarce. Even in trials that compared TAVI/SAVR in low surgical risk patients, the mean age was around 73 YO56. Therefore, although the recent European Society of Cardiology (ESC) guidelines recommend the use of surgical bioprosthetic valves in patients as young as 65 YO, it still endorses reserving TAVI for patients over 75 YO, preferring SAVR for younger patients (subject to further assessment of surgical risk, comorbidity burden, frailty, and anatomic suitability)1. The American guidelines have reduced the age threshold for considering bioprosthetic valves to 50 YO7 and its recent guidelines recommend considering TAVI in patients as young as 65 YO, pending Heart Team discussion8.
Younger AS patients have several features that distinguish them from the elderly AS population and that may affect the choice between TAVI/SAVR, the most common being a higher prevalence of bicuspid aortic valves9. In addition, it is well recognised that for bioprosthetic valves, the rate of structural valve degeneration10 becomes higher as the age at intervention decreases11.This is an issue for which data on TAVI valves are still limited. Our aim in this study was to describe the use of TAVI in younger (i.e., <70 YO) patients in "real-world" practice, examine the reasons for choosing TAVI over SAVR in this age group, and compare their characteristics and clinical outcomes with the ≥70 YO TAVI population.
Methods
PATIENT POPULATION
The Aortic+Mitral TRAnsCatheter (AMTRAC) registry (NCT 04031274) is an investigator-initiated multicentre registry of 18 TAVI centres in Europe and Israel12. The registry includes patient-level data (demographic, clinical, echocardiographic, procedural, and clinical and echocardiographic follow-up) for all consecutive patients who underwent TAVI at the participating centres. Detailed patient-level data were collected at each centre using a uniform electronic case report form and sent to the coordinating centre (Rabin Medical Center, Petah Tikva, Israel), where the unified database was compiled and analysed. For this study, we initially included all patients who underwent TAVI between 1 January 2007 and 30 June 2020 at all participating centres. For all patients undergoing TAVI younger than 70 YO as of 1 January 2015, the records of the Heart Team discussions were reviewed to identify the reason for referring these patients for TAVI rather than SAVR. We excluded patients treated prior to 2015 in order to present data relevant to recent/current clinical practice.
STATISTICAL METHODS
The percentage of patients <70 years of age out of the overall TAVI cases in the participating centres was compared across four time periods (2007-2010, 2011-2014, 2015-2017 and 2018-2020) using the chi-square test.
Patients undergoing TAVI as of 1 January 2015 were divided into two groups according to age at TAVI (
ETHICAL APPROVAL AND STUDY REGISTRATION
The registry protocol was approved by the local institutional review board as required at each participating centre, the registry is listed in ClinicalTrials.gov (NCT04031274).
Results
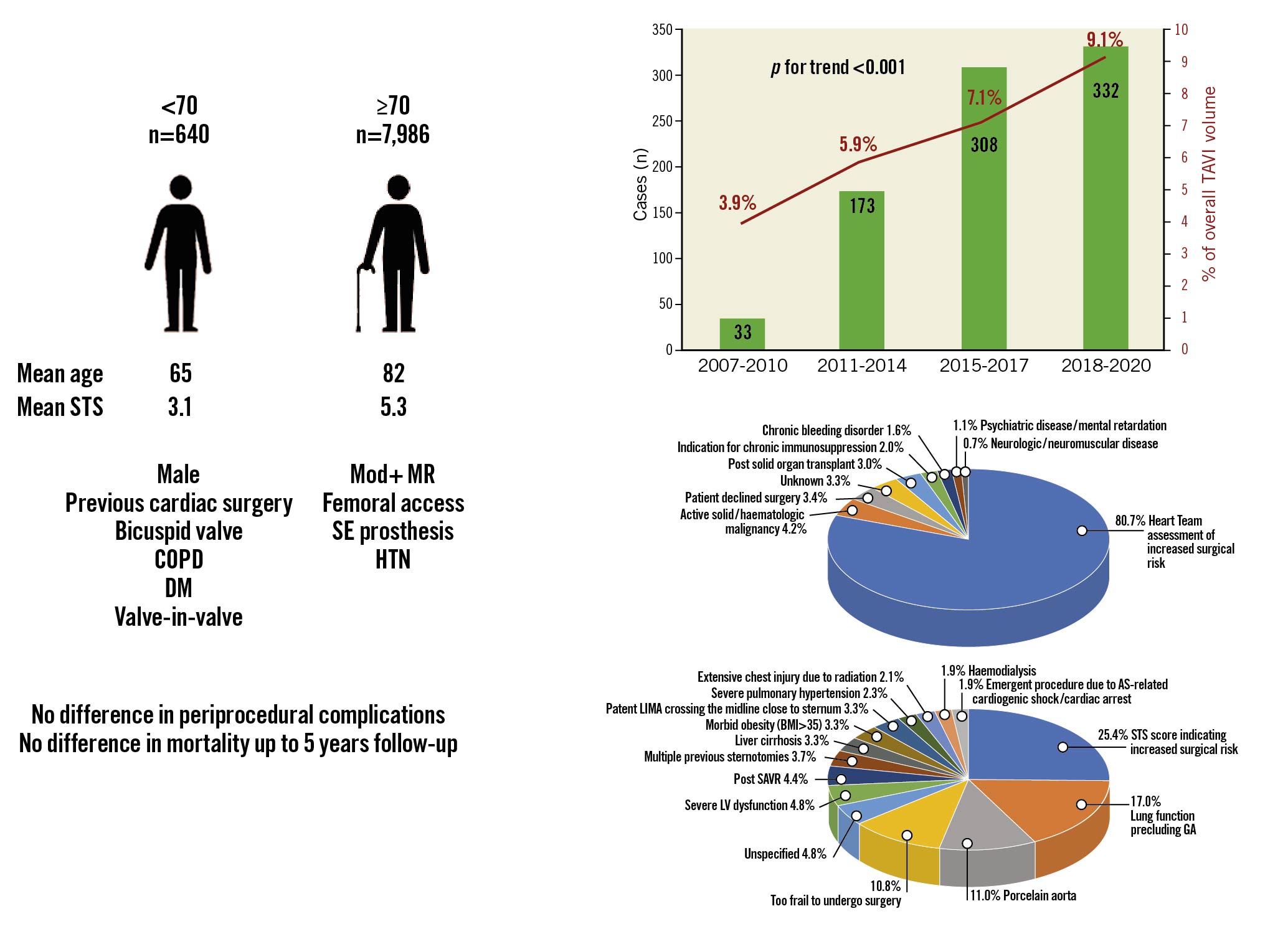
Overall, our cohort included 8,626 consecutive TAVI cases, of whom 640 (7.4%) were <70 YO. The percentage of these patients from the overall TAVI cases increased gradually from 3.9% during 2007-2010 up to 9.1% during 2018-3.2020 (p<0.001) (Figure 1).
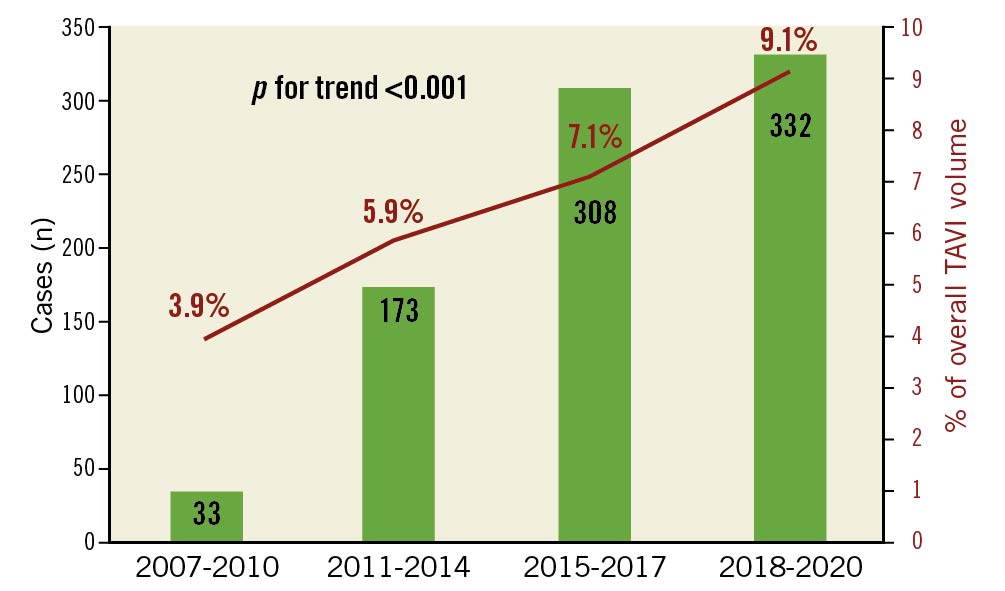
Figure 1. Change in volume of TAVI in patients <70 years and their percentage of the total TAVI volume throughout the study period. TAVI: transcatheter aortic valve implantation
Baseline characteristics of the two patient groups are presented in Table 1.
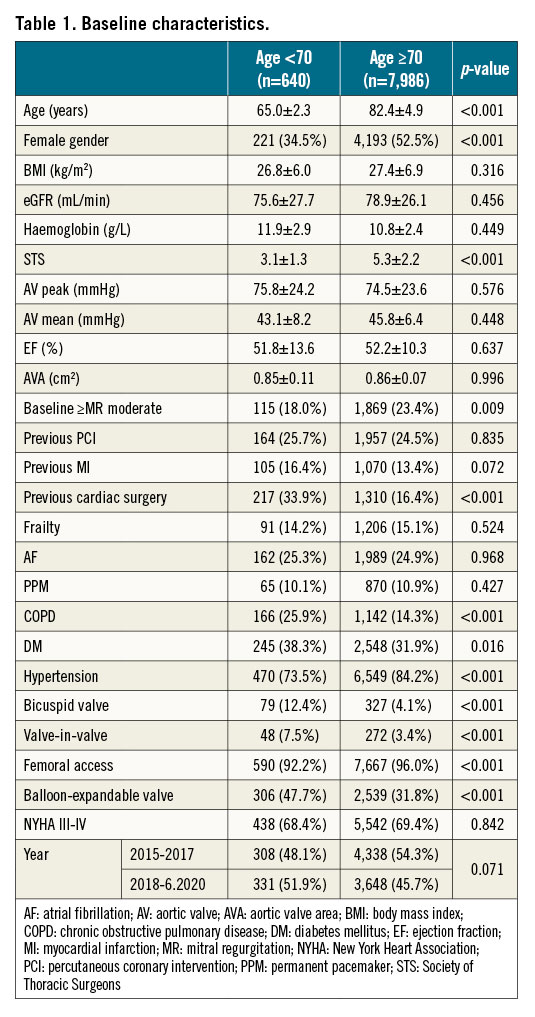
The mean age in the <70 YO group was 65.0±2.3 and 82.4±4.9 years in the ≥70 YO group. The younger patients were more likely to be male, with a history of previous cardiac surgery, chronic obstructive pulmonary disease (COPD) and diabetes mellitus, but were less likely to suffer from hypertension. For procedural characteristics, younger patients had higher rates of valve-in-valve (ViV) procedures (7.5 vs 3.4%); they were treated more often using balloon-expandable valves but were less likely to undergo the procedure via the femoral access than ≥70 YO patients (the rate of femoral access use was nonetheless very high in both groups, 92.2 and 96.0%, respectively). As expected, younger patients showed a much higher prevalence of bicuspid aortic valve (BAV) (12.4 vs 4.1%). The young patients’ group had a lower mean surgical risk according to the Society of Thoracic Surgeons (STS) score (3.1 vs 5.3, p<0.001) (Table 1).
INDICATIONS FOR TAVI IN PATIENTS <70 YEARS OF AGE
Indications for preferring TAVI over SAVR in patients <70 years of age are presented in Figure 2.
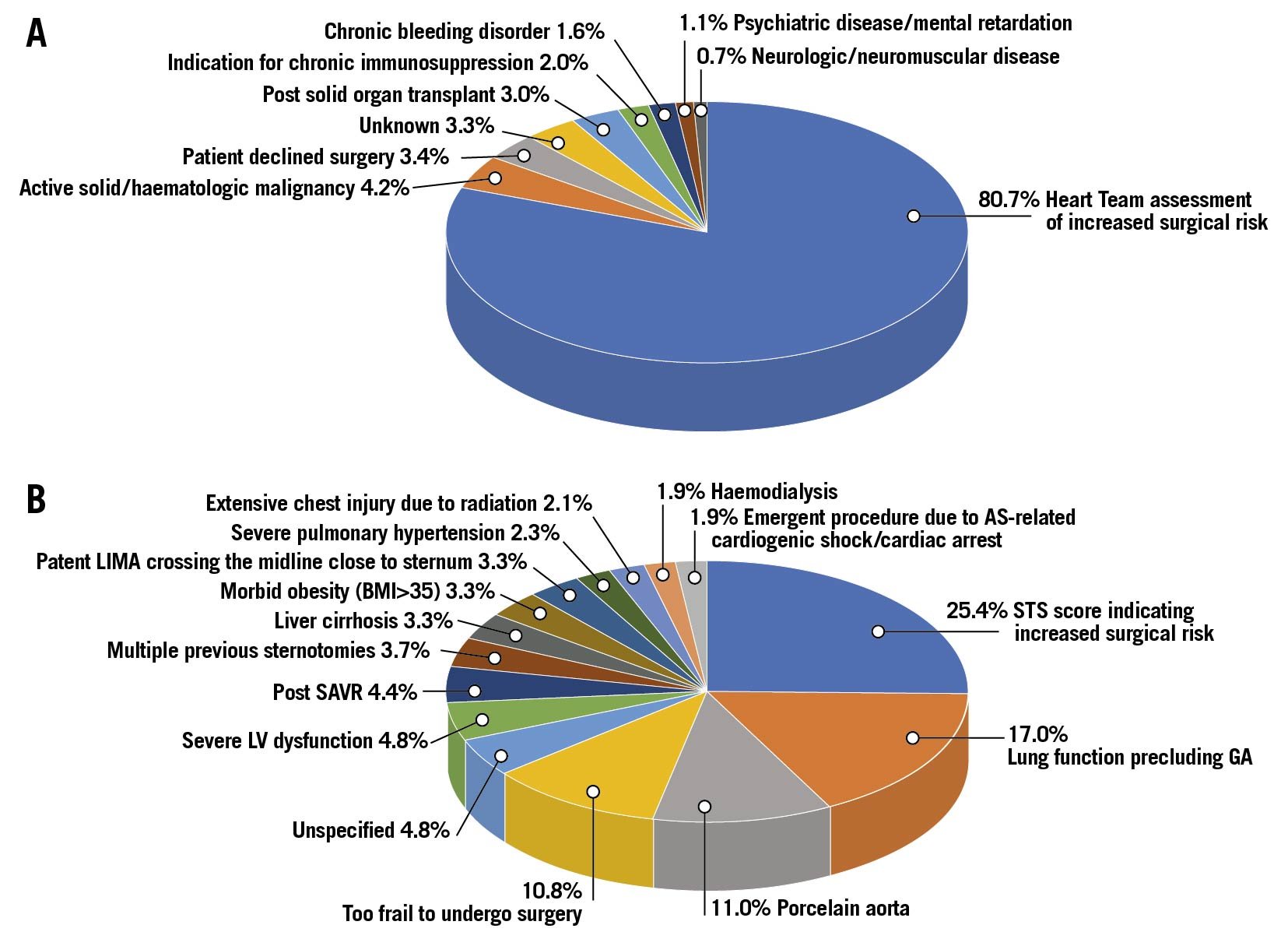
Figure 2. A) Distribution of reasons for preferring TAVI over SAVR in patients <70 years of age as specified by the local Heart Teams. B) Distribution of reasons for classifying patients <70 years of age as being at increased surgical risk as specified by the local Heart Teams. AS: aortic stenosis; BMI: body mass index; CS: cardiogenic shock; GA: general anaesthesia; LIMA: left internal mammary artery; LV: left ventricle; OHCA: out of hospital cardiac arrest; PHT: pulmonary hypertension; SAVR: surgical aortic valve replacement; STS: Society of Thoracic Surgeons; TAVI: transcatheter aortic valve implantation
The most common reason (80.7% of the overall cases) for preferring TAVI over SAVR was the Heart Team assessing the patient to be at an increased surgical risk (see detailed reasons below). Other reasons included: previous solid organ transplant or a need for chronic immunosuppression (3.0 and 2.0%, respectively), active malignancy (4.2%), chronic bleeding disorders (1.6%), psychiatric disease (1.1%) or neurologic/neuromuscular impairment (0.7%). TAVI was chosen due to the patients’ refusal to undergo surgery in only 3.4%. We were not able to ascertain the reason for preferring TAVI over SAVR in 3.3% of cases.
Of the patients deemed by the Heart Team to be at increased risk for SAVR, only 25.4% were classified as such using a threshold of STS score >4%. Other major reasons to avoid surgery were: lung function precluding general anaesthesia (17.0%), porcelain aorta (11.0%), extreme frailty (10.8%) and previous cardiac surgeries (overall 11.4% – including 4.4% previous SAVR, 3.7% multiple previous sternotomies and 3.3% patent internal mammary grafts crossing the midline close to the sternum). Less frequent reasons were echocardiographic/haemodynamic findings (overall 7.1% – including 4.8% with left ventricular dysfunction and 2.3% with severe pulmonary hypertension) and habitus/anatomy (overall 5.4% including – 3.3% with morbid obesity and 2.1% with extensive chest injury due to previous radiotherapy). Clinical presentation was the reason for increased surgical risk in 1.9% (AS-related cardiogenic shock/sudden cardiac death). In 5.2% the surgical risk was perceived as high due to comorbidities (3.3% liver cirrhosis, 1.9% chronic haemodialysis). The reason for classifying the patient as being at increased surgical risk was not available in 4.8% of cases.
CLINICAL OUTCOMES
PERIPROCEDURAL OUTCOMES
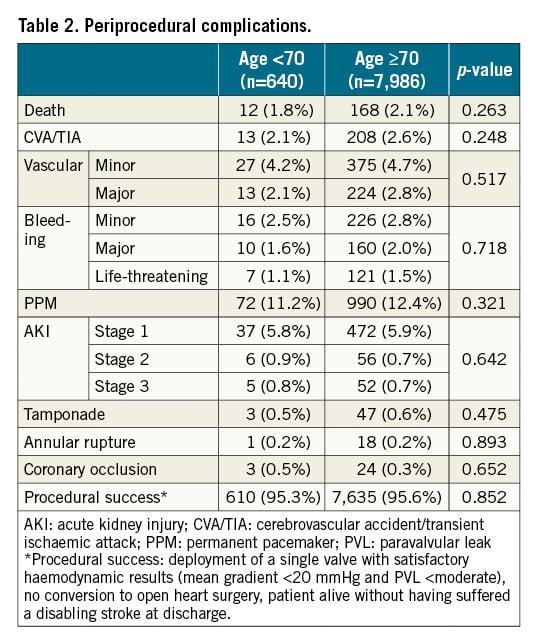
Procedural success was high and similar in patients <70 and ≥70 YO (95.3% and 95.6%, respectively). In-hospital mortality was similar (1.8% vs 2.1%, p=0.263), likewise, there was no statistically significant difference in any other periprocedural outcome (Table 2).
OVERALL MORTALITY
Overall mortality up to five-year follow-up was similar between the two groups (11.9% vs 9.7%, 24.9% vs 22.8% and 29.4% vs 29.8% at 1, 3 and 5 years for the <70 and ≥70 YO groups, respectively). This applied either when using unadjusted comparisons (log-rank p=0.132, 0.154 and 0.287, respectively), or multivariate adjusted comparison (HR=1.12, 1.06 and 0.95, p=0.432 and 0.715 p=0.287 for 1-, 3-, and 5-year mortality, respectively) (Figure 3A).
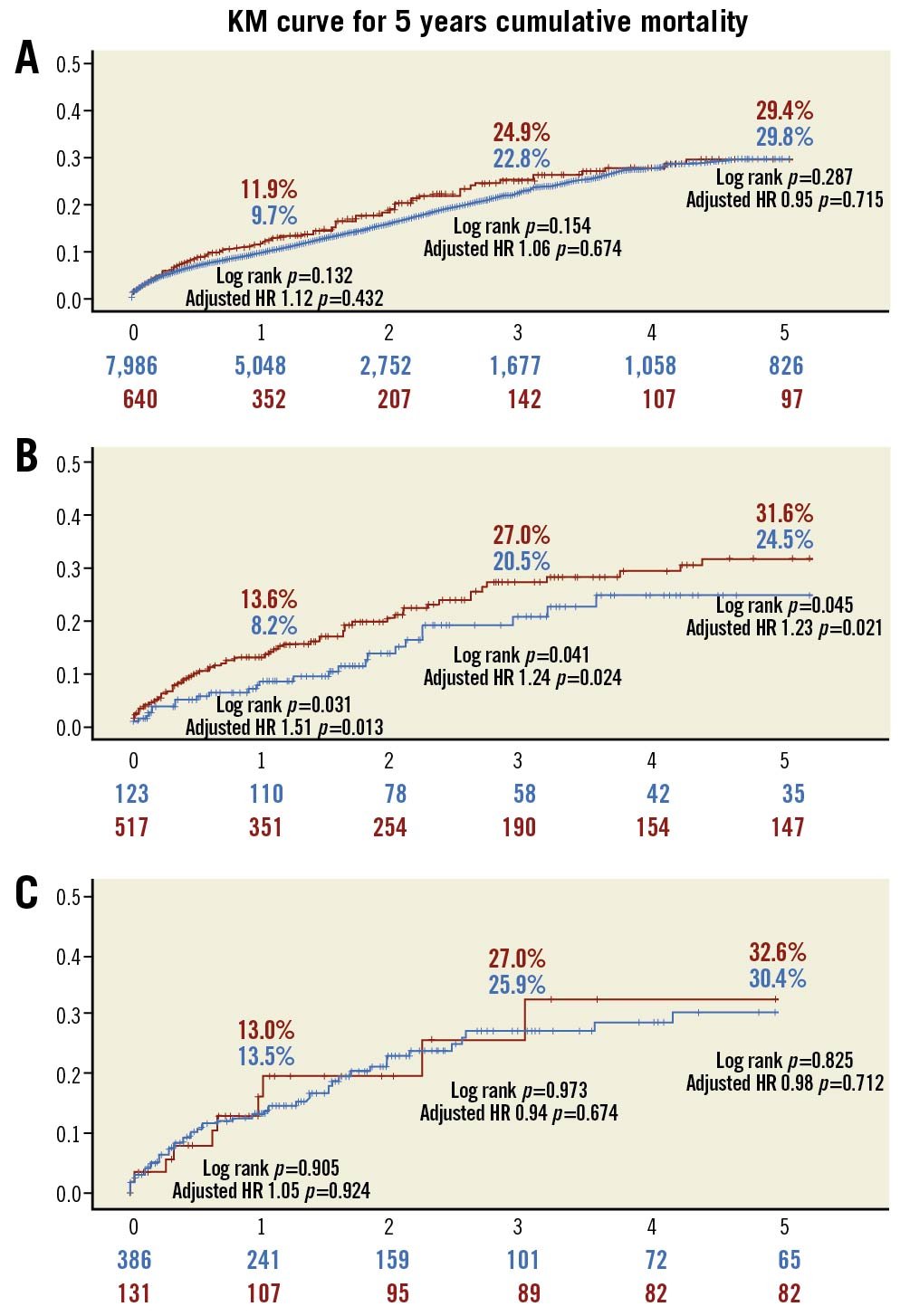
Figure 3. A) Overall mortality stratified by age at TAVI. Red: <70 years of age; blue: ≥70 years of age. B) Overall mortality in patients <70 years of age according to reason for preferring TAVI over SAVR. Red: increased surgical risk; blue: all other reasons. C) Overall mortality in patients <70 years of age referred for TAVI due to increased surgical risk according to definition of increased surgical risk. Red: STS score; blue: Heart Team assessment. HR: hazard ratio; STS: Society of Thoracic Surgeons; TAVI: transcatheter aortic valve implantation
When stratified according to the reason for preferring TAVI over SAVR (increased surgical risk vs all other reasons/unknown), the five-year mortality rates in the <70 YO group were significantly higher for those assessed by the Heart Team to be at increased surgical risk (13.6% vs 8.2%, 27.0% vs 20.5% and 31.6% vs 24.5%, 1-, 3- and 5-year, respectively). The mortality difference was statistically significant either when using unadjusted comparisons (log-rank p=0.031, 0.024 and 0.021, respectively) or multivariate adjusted comparisons (HR=1.51, 1.24 and 1.23, p=0.013, 0.024 and 0.021 for 1-, 3- and 5-year mortality, respectively) (Figure 3B).
When examining only patients <70 YO referred for TAVI due to perceived increased surgical risk, mortality was similar up to and including five years of follow-up regardless of the STS stratum (“formal” increased surgical risk as indicated by the STS score vs all others): 13.0% vs 13.5%, 27.0% vs 25.9% and 32.6% vs 30.4% 1, 3 and 5 years for the increased and low STS groups, respectively. This applied either when using unadjusted comparisons (log-rank p=0.905, 0.973 and 0.825, respectively), or multivariate adjusted comparisons (HR=1.05, 0.94 and 0.98, p=0.924, 0.674 and 0.712 for 1-, 3- and 5-years mortality, respectively) (Figure 3C).
VALVE HAEMODYNAMICS
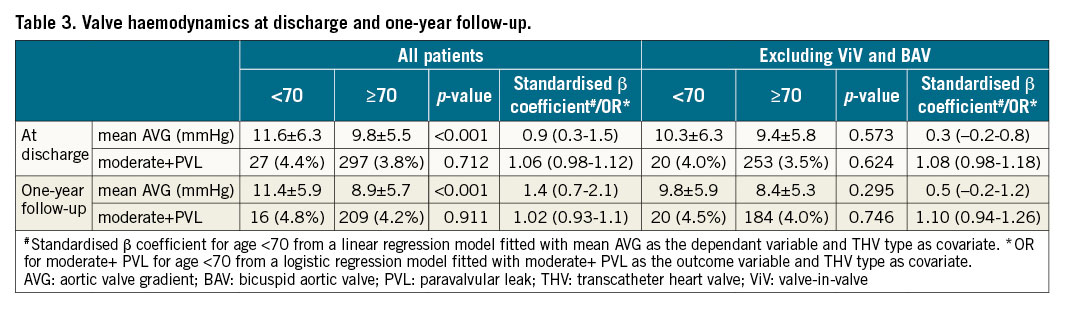
Overall, the mean aortic valve gradients were higher in patients <70 YO both at discharge and one-year follow-up, while moderate+paravalvular leak was similar regardless of age group. However, after excluding ViV and BAV cases, haemodynamic results at discharge and one-year were similar in both groups. This was also the case when excluding only ViV cases and including the BAV cases in the analysis, and also when adjusting for the difference in the distribution of THV type between the two groups (Table 3).
Discussion
The main finding of our study is that the percentage of patients <70 YO in the overall TAVI population has steadily increased over the past decade and currently constitutes 9.1% of the TAVI volume. These patients have an overall lower risk profile (as represented by the STS score) but a higher prevalence of comorbidities such as COPD, previous cardiac surgery and diabetes. They are much more likely to have bicuspid anatomy or require TAVI as a ViV procedure. By far the most common reason (80.7%) for referring young patients for TAVI over SAVR was a clinical assessment by the Heart Team that they are at increased risk for surgery (which was discordant with their STS score in most cases). Periprocedural outcomes and midterm survival of these younger patients were similar to that of the overall TAVI population. In the <70 years of age group, survival was worse for those referred for TAVI due to increased surgical risk according to the evaluation of the Heart Team as compared to other reasons for choosing TAVI (regardless of their “formal” STS risk score).
Although SAVR should be the preferred treatment for patients <70 YO according to current guidelines1, the use of TAVI in this age group is increasing rapidly, as is their percentage of the overall TAVI volume4. Currently, this practice is not backed by any clinical data, as this age group was very rare in the pivotal TAVI trials. Since the trend of expanding use of TAVI into younger and lower-risk populations is only expected to increase, presenting data on clinical outcomes of these patients in real-world settings is important and will help facilitate informed and shared decision-making by both Heart Teams and patients. To the best of our knowledge, the only previous report on TAVI outcomes of <70 YO patients comes from recently published data from a national Swiss TAVI registry that stratified TAVI patients by age groups13. During a study period starting in 2011, 324 patients (4.6% of the TAVI population) were <70 YO. The mean age and STS score of the <70 YO group was very similar to our cohort (64.6 and 3.5 compared to 65.0 and 3.1), as was the one-year mortality (11.2% compared to 11.9%), which was similar to that of the 70-89 YO group. Our data represent an international cohort with a much larger sample size, a more current clinical practice, a longer follow-up period (5 years vs 1 year) and includes detailed information on indications for preferring TAVI over SAVR. As such, its external validity is stronger and its relevance to the TAVI community greater.
Our results show that within younger TAVI patients, there is significant discordance between the Heart Team assessment and the “formal” STS score14 regarding the expected surgical risk, even though most of the factors specified by the Heart Team which place the patients at increased surgical risk are included in the STS score.
In most cases, the Heart Team’s preference for TAVI over SAVR was driven by conditions/comorbidities that can generally be classified as relative contraindications for surgery (even conditions such as porcelain aorta and lung function precluding general anaesthesia do not have a uniform definition and may be viewed differently in different institutions according to local practice/expertise). These data highlight the limited usefulness of the STS score, developed using data on patients undergoing SAVR, for risk stratification of TAVI candidates, and raise the question: what is the source for this discordance? We believe this is due to the profound and disruptive effect that the introduction and rapid expansion of TAVI over the past decade has had on the SAVR population. Data from the recent STS Adult Cardiac Surgery Database and the Transcatheter Valve Therapy (TVT) Registry annual reports415, reveal that while the annual volume of isolated SAVR in the United States has been steadily declining, the annual TAVI volume increased from around 16,000 in 2014 (of whom 95% were at high surgical risk/inoperable) to 72,000 cases in 2019. Among these, 11% were at low surgical risk, with the rest evenly divided between intermediate and high/inoperable risk). This would suggest that the introduction of TAVI into clinical practice resulted in the majority of intermediate-/high-risk patients being referred for TAVI (consistent with the current ESC and American guidelines), leaving low-risk patients as the main population currently undergoing SAVR. This is also supported by the STS database statistics on 30-day mortality post SAVR (the outcome approximated by the STS score) – which was 1.9% in 2019 (corresponding to the mid-range of the low-risk stratum)15 – and a multicentre registry of isolated SAVR procedures in the United States in 2013-201716. Under these circumstances (e.g., low-risk patients as the reference in everyday practice and thus less exposure to more challenging patients compared to the pre-TAVI era), it becomes challenging to train and maintain proficiency in performing complex surgical cases. This is expected to make surgeons more reluctant to accept patients with high-risk features, either clinical (emergent procedures, previous sternotomies, patent arterial grafts crossing the midline, morbid obesity, liver cirrhosis, haemodialysis) or haemodynamic (left ventricular dysfunction, pulmonary hypertension) for SAVR, and more likely to refer these complex cases for TAVI, regardless of their age.
Unlike Heart Team discussions regarding myocardial revascularisation, where evidence on reproducibility17 as well as the prognostic effect of discordance between the Heart Team decision and practice guidelines18 have been published, no such data exist for Heart Teams regarding the treatment of valvular heart disease. Since we have no data on young AS patients with similar baseline characteristics referred for SAVR and cannot compare their outcomes to our cohort of young patients undergoing TAVI, we cannot examine whether the SAVR outcomes would have been different, thus assessing the adequacy of the Heart Teams’ decisions. However, the notion that the surgical risk of many of the young patients in our cohort was underestimated by the formal STS score is supported by two findings. First, our outcome data showing that overall mortality in our younger patients group was similar to that of the “standard” TAVI group in our cohort (who had a mean STS score higher by 2.7% and were on average 17 years older). Second, the similar mortality of patients with STS scores indicating “formal” increased surgical risk to those with lower STS scores judged by the Heart Team to be at increased surgical risk for other reasons.
These data demonstrate the importance of individual Heart Team discussions to identify at-risk populations not captured by standard risk scores.
The main message from our study is that younger patients (<70 YO) referred for TAVI in current/recent clinical practice, are a unique group in terms of their baseline characteristics. As such, they cannot be assumed to represent the overall AS population of this age group, nor should their outcomes form the basis for decisions regarding expanding the indication for TAVI into younger age groups. Our data stress the need for randomised trials to compare SAVR/TAVI in the overall AS population under 70 YO in order to inform therapeutic strategies, which carry significant implications in terms of health economics, resource utilisation and planning of care for patients suffering from severe AS.
Our study has several strengths: it is the first large-scale report on patients in this age group undergoing TAVI with access to detailed information from the Heart Team discussions in the participating centres, and represents real-world data from a large international multicentre registry of contemporary TAVI practice.
Limitations
We could only compare the outcomes of the young AS cohort to “standard” TAVI patients, not those of similar-age patients undergoing SAVR (see above): the STS scores were calculated at each site; data on the reason for choosing TAVI over SAVR were missing in 3.3% of cases; our data are derived from European and Israeli centres only, mostly operating within a public national healthcare system, and may not be representative of other settings; the follow-up period of 5 years is relatively short for young patients; we do not have data on specific outcomes relevant to younger patients such a patient prosthetic mismatch19 or need for coronary intervention20 following TAVI; finally, we cannot present robust data regarding haemodynamic results beyond the one-year follow-up, since echocardiographic data beyond this timepoint were unavailable for the vast majority of patients.
Conclusions
The percentage of patients younger than 70 years of age amongst the overall TAVI population is growing. The choice of TAVI over SAVR is most often due to an assessment of increased surgical risk, not adequately represented by the formal STS score. The midterm outcomes of patients younger than 70 years of age are similar to those of the overall TAVI population – in spite of being, on average, 17 years younger (Central illustration). Dedicated trials comparing TAVI with SAVR in the all-comers young AS population are needed to guide decisions regarding further expansions of TAVI indications to younger age groups.
Central illustration. Left panel: Primary differences between the
Impact on daily practice
In the first large-scale study of patients <70 years of age undergoing TAVI (with a mean age 65), we found that these patients exhibit a unique comorbidity profile, setting them apart from the overall AS population of similar age. In most cases, the Heart Team’s assessment was that the formal STS score underestimated the surgical risk. Clinical outcomes for these younger patients were similar to the overall TAVI population (who are, on average, 17 years older). Our results highlight the limitations of the STS score in assessing procedural risk in young AS patients, and the importance of Heart Team discussion. More data (ideally a randomised trial) comparing TAVI vs SAVR in the overall AS population younger than 70 years of age are needed.
Appendix. Authors’ affiliations
1. Division of Cardiology, Rabin Medical Centre, Petah-Tikva, Israel; 2. The Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel; 3. Division of Cardiology, University of -Catania, Catania, Italy; 4. The Heart Center, Rigshospitalet, -Copenhagen University Hospital, Copenhagen, Denmark; 5. Department of Cardiology, Thoraxcenter, Erasmus University Medical Center, Rotterdam, the Netherlands; 6. Cardiovascular Institute, Hospital Clinico San Carlos, IdISSC, Madrid, Spain; 7. Department of Cardiac, Thoracic and Vascular Sciences, University of Padua Medical School, Padua, Italy; 8. Division of Cardiac Surgery, Medical University of Vienna, Vienna, Austria; 9. Department of Cardiology, University Hospital Galway, National University of Ireland, Galway, Ireland; 10. Cardiovascular Department, Spedali Civili, Brescia, Italy; 11. Hospital de Sant Creu i Sant Pau Barcelona, Barcelona, Spain; 12. CIBERCV, Hospital Clínico Universitario de Valladolid, Valladolid, Spain; 13. Servicio de Cardiología, Hospital Álvaro Cunqueiro, Vigo, Pontevedra, Spain; 14. Department of Cardiology, University Medical Centre, Ljubljana, -Slovenia; 15. Department of Cardiology, Heart Centre, Faculty of Medicine, University of Cologne, Cologne, Germany; 16. Tel Aviv Sourasky Medical Center, Tel Aviv, Israel; 17. Department of Cardiology and Angiology II, University Heart Center Freiburg-Bad -Krozingen, Bad Krozingen, Germany; 18. San Camillo Forlanini Hospital, Rome, Italy; 19. Herzzentrum Bonn Universitätsklinikum Bonn, Bonn, Germany
Conflict of interest statement
N.M. Van Miegham received research grant support from Abbott, Boston Scientific, Edwards Lifescience, Medtronic, PulseCath BV and Daiichi Sankyo, advisory fees from Abbott, Boston Scientific, Ancora, Medtronic, PulseCath BV and Daiichi Sankyo. M. Barbanti received consultant fees from Edwards Lifesciences. C. Grasso is a proctor for Abbott Vascular. O. De Backer received research grants and consultant fees from Abbott and Boston Scientific. M. Andreas is a proctor/consultant for Abbott, Medtronic and Edwards Lifesciences, received institutional grant support from Edwards, Abbott, Medtronic and LSI. R. Estévez-Loureiro is a consultant for Abbott Vascular and Boston Scientific. L. Nombela-Franco received consultant fees from Edwards Lifesciences and is a proctor for Abbott. L. Sondergaard received consultant fees and institutional research grants from Abbott, Boston Scientific, Edwards Lifesciences and Medtronic. I. J. Amat-Santos is a proctor for Boston Scientific. M. Bunc is a proctor for Edwards, Medtronic, Abbott, and Meril and is on an advisory board for Medtronic. M. Adam received consultant fees from Medtronic, Edwards Lifescience and Boston Scientific. The other authors have no conflicts of interests to declare.
Supplementary data
To read the full content of this article, please download the PDF.

