Abstract
Aims: In contrast to the cytostatic and cytotoxic pharmacotherapy of drug eluting stents, approaches to stimulate the arterial healing response by accelerating re-endothelialisation may be an attractive alternative to prevent post-stenting neointimal formation and thrombosis. The study sought to evaluate whether stainless steel stents able to sequester endothelial progenitor cells (EPCs) to the stent surface to promote the endogenous arterial healing, can prevent in-stent restenosis and stent thrombosis.
Methods and results: The HEALING-II study was a multicentre, prospective registry study, including 63 patients treated with the implantation of a Genous™ EPC capture stent. The primary safety endpoint was Major Adverse Cardiac Events (MACE) at 30 days, whereas the primary efficacy endpoint was percent in-stent volume obstruction at 6 months by intravascular ultrasound (IVUS).
The 9 month composite MACE rate was 7.9%, whereas 6.3% clinically justified target lesion revascularisations were observed. Although patients received one month of clopidogrel, no angiographic stent thrombosis occurred. In-stent late luminal loss was 0.78±0.39 mm and percent in-stent volume obstruction was 22.9±13.7% (mean±sd). Patients with normal EPC titers at 6 months FU responded favourably to the EPC capturing stent (late luminal loss 0.53±0.06) as opposed to patients with low EPC titers (late luminal loss 1.01±0.07). These low circulating EPC titers were generally associated with the lack of HMG-Co-A reductase inhibitors (‘statins’) at the time of stent implantation and diabetes mellitus.
Conclusions: The HEALING-II study suggests that the EPC capture coronary stents are safe and effective for the treatment of de novo coronary artery disease in patients with normal EPC levels.
Introduction
The introduction of drug eluting stents in the coronary interventional lab has dramatically reduced the incidence of in-stent restenosis and improved the outcome and efficacy of complicated multivessel PCI. The DES-associated pharmacotherapy is generally aimed to induce cytostasis or cytotoxicity of the inferring hyperproliferative vascular smooth muscle cells that give rise to neointimal hyperplasia. Thereby, these antiproliferative drugs impede the natural healing response by delaying the formation of a functional endothelial lining over the stent, giving rise to an increased incidence of stent-related thrombosis and vasomotor dysfunction, as evidenced by a paradoxical or absent vasoconstrictive response to acetylcholine in the proximal and distal segment of DES treated coronary arteries1,2.
Circulating endothelial progenitor cells have been shown to play a crucial role in the initiation of the arterial repair response by governing re-endothelialisation following arterial injury by balloon angioplasty. These circulating endothelial progenitor cells are also upregulated in vascular disease including atherosclerosis, diabetes, and after myocardial infarction3-5 suggestive of ongoing vascular repair and endothelial turnover. In the EPC capture stent technology at hand, circulating CD34+ progenitor cells are sequestered to the luminal stent struts to differentiate into a functional endothelial layer devoid of endothelial dysfunction and capable of preventing stent-related restenosis and thrombosis.
The EPC capture stent (Genous™ R stent, OrbusNeich MT) has been developed using immobilised anti-human CD34 antibodies targeted an EPC cell surface antigen. Our previous studies have shown that CD34 antibody-mediated EPC sequestration led to a remarkable rapid and efficient differentiation into a fully functional endothelial lineage both in vitro and in vivo in a porcine animal model14. The HEALING-first in man registry demonstrated the safety and feasibility of an EPC capturing stent technology in 16 cardiovascular patients leading to a overall late luminal loss of 0.63±0.52 and a binary restenosis rate of 13.3%, without concomitant stent-related thrombotic events6. The concept of an EPC capture mediated accelerated arterial repair response was further explored in the multicentre, prospective, non-randomised HEALING-II clinical registry enrolling 63 patients with de novo coronary artery disease. Risk factors for coronary artery disease have been shown to reduce the number and functional activity of these circulating EPCs, thus limiting their regenerative capacity5,7,8. The impairment of circulating endothelial progenitor cells by cardiovascular risk factors may contribute to the limited regenerative capacity in cardiovascular patients rendering them more prone to restenosis formation and atherosclerotic disease progression. Therefore, circulating EPC levels in study patients was cross-correlated with angiographic and IVUS outcome at 6 months follow-up to designate patient population most likely to respond to the EPC capturing stent technology.
Materials and methods
Patient population selection
A total of 63 patients were enrolled throughout 10 medical centres in the Netherlands, Belgium and Germany. Patients were eligible for enrolment if they were between 18 and 85 years old and had a diagnosis of de novo stable or unstable angina or silent ischaemia based on a single, de novo lesion of a native coronary artery eligible for coronary stenting. The target lesion had to be less than 12 mm in length, able to be covered by a single trial stent (13 mm or 18 mm length), whereas the reference artery should measure between 2.5 and 3.5 mm vessel diameter by visual estimate.
Exclusion criteria for study participation included an evolving myocardial infarction within 72 hours preceding the index procedure; renal dysfunction or suspected liver disease; thrombocytopenia or leucopenia; unprotected left main coronary artery disease; ostial target lesions; severely calcified lesions; totally occluded vessels, excessive tortuousity of the target vessel and target lesions involving a bifurcation with a side branch that would require stenting; intervention of another lesion within 6 months before or anticipated within the scheduled follow-up of the index procedure; or left ventricular ejection fraction of less than 30 percent. The trial was reviewed and approved by the local medical ethics review committee, and written informed consent was obtained from all patients.
Endothelial progenitor cell capture stent
Endothelial Progenitor Cell Capture Stent is comprised of a stainless steel stent (9 or 18 mm R stent™, OrbusNeich Medical Technologies, Fort Lauderdale, FL, USA) coated with an adluminal polysaccharide matrix and covalently coupled monoclonal murine anti-human CD34 antibodies. These anti-CD34 antibodies specifically target the circulating endothelial cell progenitor population associated with post-natal neoangiogenesis and arterial repair response. Whereas the study device in the previous HEALING-FIM registry required hand crimping by the operator onto a PTCA balloon catheter, the current study device, is comprised of a dry preparation CD34-antibody coAted R stent™ premounted on an Evolution2™ PTCA balloon catheter.
Quantitative angiographic and ultrasound analysis
A qualifying angiogram was used to assess angiographic in- and exclusion criteria. Following verification of all angiographic and medical in- and exclusion criteria, patients signed the informed consent and were enrolled in the study. Coronary intervention was performed according to standard local institutional protocols.
Pre-dilation of the target lesion was left at the investigator’s discretion, whereas post-dilation was performed as required to achieve less than 20% residual stenosis by visual assessment, with TIMI grade III flow.
Stent deployment was performed at nominal pressure as recommended, to a stent-to-vessel ratio of 1.1:1. Following stent placement, the IVUS catheter was introduced and an automated pullback was performed. The procedure was deemed angiographic successful when residual diameter stenosis within the stent was less than 20% in the presence of grade III TIMI flow, as measured by on-line QCA and the mean reference diameter of the stent was larger than 1.1 with respect to the reference diameter of the segment post-stenting (e.g. interpolated reference diameter). Procedural success was defined as an angiographic successful stent placement and the absence of cardiac death, MI, CABG or a Target Lesion Revascularisation event during the hospital stay.
Treatment with aspirin, at a dose of 80-150 mg per day, was initiated at least 12 hours before the procedure and continued for six months post-intervention. In addition, a loading dose of 300 mg of clopidogrel was administered prior to the procedure, followed by 75 mg daily for 4 weeks. Administration of GPIIb/IIIa was left to the investigator’s discretion according to clinical practice.
Coronary angiograms were obtained in perpendicular views following an intracoronary injection of nitrates. Off-line quantitative analyses of preprocedural, post-procedural and six month follow-up angiographic data were performed at an independent imaging core laboratory (Cardialysis, Rotterdam, The Netherlands). Restenosis was defined as a reduction of at least 50 percent of the luminal diameter. Late luminal loss was defined as the difference between the minimal luminal diameter after procedure and at six months follow-up. The target lesion was defined as the stented segment plus the 5 mm segments proximal and distal to the stented segment.
Intravascular ultrasound (IVUS) was performed with an automated pull back at 0.5 mm/s to examine the target lesion at post-procedure and 6-month follow-up. Lumen, stent, and external elastic membrane contours were detected with the use of CUARD QCU analysis software (CUARD BV, Wijk Bij Duurstede, NL), applying a 3-dimensional reconstruction, as described elsewhere, and submitted for off-line analysis by the independent IVUS core laboratory (Cardialysis, Rotterdam, NL). Proper stent apposition to the target vessel wall was verified by visual assessments by independent investigators of the IVUS core laboratory.
Follow-up
All patients were scheduled for a clinical follow-up at 1, 6, 9 and 18 months following the implantation procedure to assess the anginal status (according to the Canadian Cardiovascular Society Classification of angina and the Braunwald Classification for unstable angina) and the documentation of MACE (including any interventional treatment [e.g., repeat TLR] and serious adverse events). The results of the 18 month angiographic and clinical follow-up will be reported separately. Angiographic and IVUS study was performed at 6 months follow-up. HAMA titer analysis was performed at baseline, 1 and 6 months post-intervention.
Study endpoints
The primary safety endpoint of the study was defined as a composite of MACE at 30 days defined as the incidence of cardiac death, Q-wave or non-Q-wave myocardial infarction, emergent cardiac surgery and clinically justified target lesion revascularisation (TLR) within 30 days following the index procedure. A TLR/TVR was considered clinically justified when the diameter stenosis was more than 50% (by off-line QCA) and either, (1) a history of recurrent angina pectoris, or (2) ischaemia related ECG changes at rest or during exercise test related to the target vessel or either (3) an abnormal result of any invasive functional diagnostic test (including Doppler flow velocity reserve or fractional flow reserve). A TLR/TVR with a diameter stenosis > 70% (by off-line QCA) in the absence of the aforementioned ischaemic signs or symptoms was also considered clinically driven. The primary efficacy endpoint was % in-stent volume obstruction at 6 months as assessed by off-line IVUS.
Secondary safety endpoints comprised MACE rate and device-related SAEs until 9 months follow-up and the incidence of stent thrombosis as documented by coronary angiography. An independent Clinical Events Committee adjudicated all major adverse cardiac events. A data safety monitoring board reviewed safety data on a regular basis. Secondary efficacy endpoints included QCA derived vessel parameters of the in-stent segment and 5 mm proximal and 5 mm distal from the edge of the stent: including acute gain, MLD, mean diameter, % diameter stenosis and binary restenosis rate, angiographic and procedural success, clinically justified TLR free rate at 6 months, and quantitative IVUS assessment, including proper stent apposition. Stent thrombosis was defined as an angiographically documented complete occlusion or a flow limiting thrombus (TIMI flow 1 or 2) of a previously successfully treated artery.
Detection of serum human anti mouse antibody titer
Human anti mouse antibody (HAMA) titer was determined at the time of intervention, one and six months post-intervention for each patient treated in the HEALING-II Registry. Testing was conducted using a commercially available photometric ELISA for the quantitative determination of HAMA from patient blood (Roche Diagnostics). HAMA titers of > 10 ng/ml was considered positive, whereas HAMA titers exceeding 150 ng/ml was considered to be significantly elevated.
Analysis of circulating CD34+/KDR+ endothelial progenitor cells by fluorescence aided flow cytometry
The efficacy of the EPC stent to accelerate arterial repair and prevent coronary neointimal hyperplasia could be affected by the titer and functional properties of the endogenous circulating EPCs. To quantify the circulating endothelial progenitor cells (EPCs) by quantitative flow cytometric analysis in the patients, at 6 months follow-up, whole blood was incubated with 7 AAD viability dye, APC-labelled anti-hCD34, FITC labelled anti-hCD45 (Pharmingen) and PE-labeled anti-hKDR (R&D systems) according to the manufacturer protocol (and ISHAGE guidelines) for 20 minutes at room temperature using Stem-Kit™ reagents (Beckman Coulter Comp, Cedex, FR). After addition of counting fluorospheres (Beckman Coulter, Cedex, FR), the red cell fraction was lysed by NH4Cl and analysed on an automated flow cytometer (FACSCanto®, Becton&Dickenson). Corresponding isotypic IgG1 controls were used to set proper gating for each antibody. Whole blood samples were analysed by flow cytometry within 24 hours after collection at the different sites. Endothelial progenitor cell (EPC) titer was expressed as absolute number of viable EPCs per 100 µl of whole blood (7AAD–/ CD45+/CD34+/KDR+).
Statistical analysis
The HEALING-II study was designed as an exploratory, prospective, non-randomised trial. No prior formal power calculations were conducted to determine the efficacy of the EPC capture stent technology. The primary analysis was performed according to the intention to treat principle and included all patients initially enrolled in the study. Continuous various variables are summarised by mean±standard deviation. Post-procedural and 6 month follow-up angiographic and IVUS changes were compared using a 2-tailed paired t-test. P<0.05 was considered to be statistically significant. Only events adjudicated by the Clinical Event Committee have been taken into account for the analysis of MACE.
Results
Baseline characteristics and procedural outcome
Overall, 63 patients were included in the HEALING-II registry between May and October 2004. Table 1 presents the detailed baseline characteristics and procedural outcomes for this patient population.
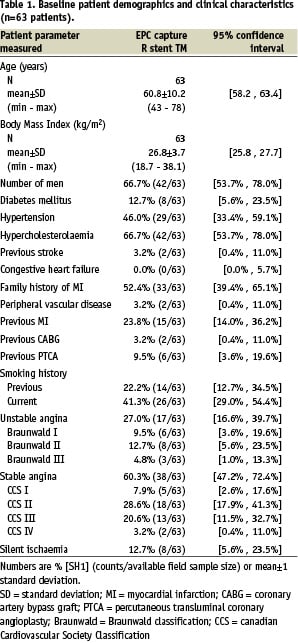
Baseline demographics and clinical characteristics showed that patients had a mean age of 61 years, 66.7% male participation and 12.7% suffered from diabetes mellitus.
Predilatation of the target lesion was performed in 41 out of 63 patients. Overall angiographic and procedural success were both 93.7% (59/63; Table 4).
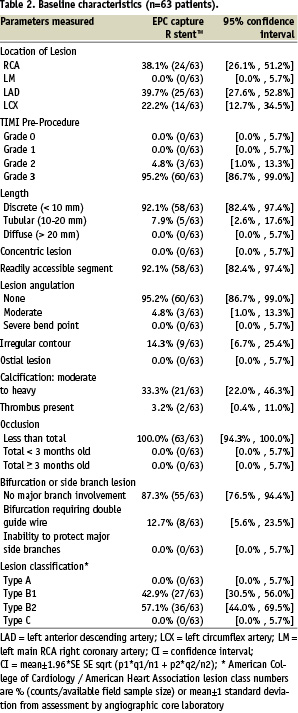
Further stent expansion using post dilation at higher pressures to achieve optimal sizing was required in 25 out of 62 cases. (< 20% residual stenosis with TIMI III flow). In-stent malapposition as measured by IVUS, occurred in 0 out of 62 patients. In one patient the delivery system could not cross the target lesion and eventually a conventional bare metal stent was implanted (1/63). A second overlapping EPC capture stent was implanted in four patients to treat a dissection following implantation of the first stent, and in one patient due to incomplete coverage of the target lesion by the first stent.
Clinical outcome
Compliance to clinical follow-up at 240 days was 98.4% and at 270 days it was 87.3%. Table 3 provides an overview of the MACE at 1 and 9 months.
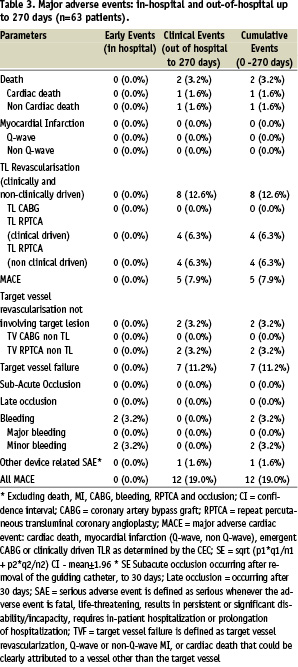
No acute or subacute thrombosis occurred in patients over the 9 month time period despite one month of dual antiplatelet therapy. One patient died at day 90 post-implantation, after falling down the stairs at home. As the event was without witnesses and permission for autopsy was not acknowledged, cardiac death could not be excluded. Therefore, this patient was adjudicated as cardiac death. At 1 month clinical follow-up, this patient has been event and angina free.
Another patient died at day 270 days following stenting after being diagnosed with ostial and pulmonary metastasised synovial sarcoma, 3 months following the index procedure. The patient was adjudicated by the CEC as a non-cardiac death, not associated with the investigational study device. Myocardial infarction did not occur in any of the patients during the 9 month follow-up.
Target lesion revascularisation (TLR) by repeated PCI occurred in 8 out of 63 patients (12.6%), of which 4 were adjudicated clinically driven (6.3%). Revascularisation was based on ischaemic symptoms (3/4), a positive stress test (1/4), and ischaemic ECG changes (1/4). An angiographic stenosis of > 70%, as an angiographic indication for TLR, did not occur in any of the patients. Target vessel revascularisation (TVR) by repeated PCI occurred in 2 out of 63 patients (3.2%). Total adjudicated MACE rate (including cardiac death, myocardial infarction, CABG, clinically driven TLR) of the EPC capture stented patients was 7.9%. All clinical driven and non-clinical driven MACE (including all death, myocardial infarction, CABG, total TLR and TVR) was 19.0% (Table 3).
Six-month angiographic and IVUS analysis
of target vessel
Compliance to angiographic follow-up at 6 months was 92.1% (58/63 patients). At 6 months follow-up, in-stent MLD was 1.67±0.51 mm (post-procedural in-stent MLD 2.45±0.35), whereas late luminal loss was 0.78±0.39 mm on QCA (mean±SD; Table 4).
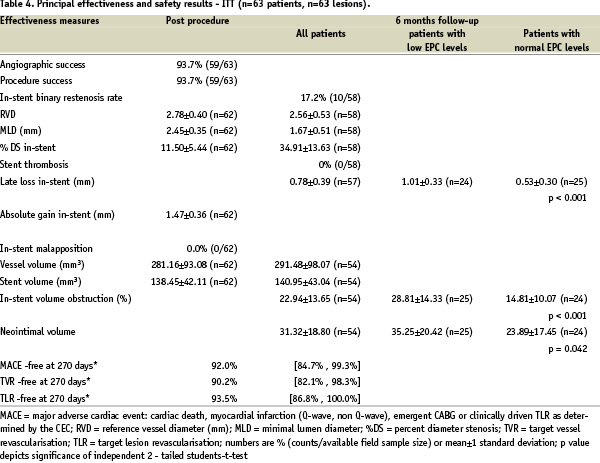
The late luminal loss index as defined by the quotient of late luminal loss and absolute gain was 0.55±0.28. The in-stent binary restenosis rate at 6 month angiographic follow-up was 17.2% (10/58 patients). Overall % diameter stenosis by QCA was 34.9%±13.6%.
IVUS analysis did not reveal malapposition of the stent in any of the study patients. In-stent volume obstruction as assessed by IVUS was 22.9±13.7% (plaque volume 31.3±18.8 mm3; plaque behind stent volume 150.5±62.0 mm3).
Flow cytometric analysis of EPC titer
Angiographic and IVUS outcome closely correlated with circulating EPC titers. EPC titer was analysed in 49 out of 58 patients at the 6 months angiographic follow-up (Figure 1).
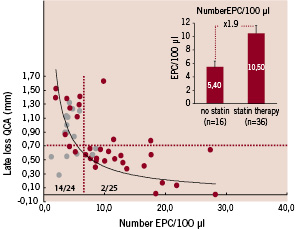
Figure 1. Cross correlation between EPC titer and angiographic late luminal loss. Use of HMG-CoA-reductase inhibitors at inclusion and stent implantation in patient population cross-correlated with EPC levels at 6 month follow-up (yellow marker: treatment with HMG-CoA-reductase; purple marker: no treatment with HMG-CoA-redutase). Insert: mean EPC levels of patient population treated with and without HMG-CoA-reductase inhibitors at the time of stent implantation (mean±sem).
Low EPC titer (EPC < 6.5/100 µl whole blood) was associated with significant late luminal loss and poor clinical outcome as compared to patients with normal EPC titers (late luminal loss: low EPC titer (< 6.5/100 µl): 1.01±0.07 vs normal EPC titer (> 6.5/100 µl): 0.53±0.06 (mean±sem); F(40,1)=5.22, p<0.001). In addition, all TLR and TVR events at 6 months time follow-up were restricted to the patient group with reduced EPC titers (6/24 (low EPC levels) vs 0/25 (normal EPC levels), p=0.008, Mann-Whitney-U test). IVUS sub-analysis likewise suggested that low EPC levels were associated with a % volume obstruction comparable to bare metal stenting, whereas normal EPC levels were associated with a reduced %volume obstruction (28.8±2.87% (low EPC titer;) vs 14.8±2.87% (normal EPC titer; mean±sem); p<0.001).
Although diabetes was more prevalent in the patient group with low EPC titers, as compared to normal EPC titers (5/24 vs 2/25, p=0.204, Mann-Whitney-U test), other CAD risk factors were not significantly correlated with reduced levels of EPCs. In contrast, the use of HMG-coA-reductase inhibitors (statins) at the time of stent implantation was significantly correlated with EPC levels, and hence, angiographic and IVUS outcome (Figure 1). In the patient group with reduced number of EPCs, the majority of the patients did not use any form of statin therapy at the time of EPC stent implantation (14/24) as compared to patients with normal EPC levels (2/25; p=0.001, Mann-Whitney-U test). In addition, statin therapy was associated with a 1.9-fold increase of EPC number (5.4±0.94 vs 10.5±1.12; (mean±sem); p=0.001) and improved angiographic outcome (late luminal loss index: 0.65±0.05 vs 0.50±0.05, (mean±sem); p<0.001). These data suggest that reduced EPC levels are associated with poor clinical and angiographic outcome of the EPC capturing technology to prevent restenosis, comparable to BMS. In addition, statin therapy may improve the EPC titer and hence, the efficacy of EPC stent to reduce neointimal hyperplasia formation in CAD patients.
HAMA test results
HAMA testing was performed at baseline, 1 and 6 month follow-up. Treatment with the murine antibody coated EPC stent resulted in a moderate and transient rise of HAMA titer in only 2 out of 63 patients tested. HAMA titer in these patients returned to baseline levels at 6 month follow-up. None of the patients experienced any phenomena indicating an allergic reaction to the product including shivering, fever or rash.
Discussion
Main findings
The HEALING-II registry was a prospective, multicentre clinical registry to assess the safety and efficacy of the first bioengineered stent aimed to accelerate the endogenous arterial healing response by capture of circulating EPCs to the stent. Various animal and human studies have shown mobilisation of circulating EPCs and CD34+ cells with endothelial damage, vascular trauma and acute myocardial infarction, reflecting increased endothelial cell turnover and ongoing vascular repair9. These EPCs may provide a circulating pool of cells that could form a cellular patch at the site of denuding injury or serve as a cellular reservoir to replace dysfunctional endothelium. We were able to develop (in collaboration with OrbusNeich) a tissue engineering approach to promote cell seeding and re-endothelialisation of the stent by a proprietary technology to bind anti-CD34 antibodies to the luminal surface of the stent. This CD34 antibody is directed against towards the surface markers of circulating EPCs and will sequester these progenitor cells to the stent surface within minutes to hours. In vitro and in vivo studies have shown that these sequestered cells differentiate in fully functional endothelial cells. In the pig balloon injury model, EPC capture stent implantation indeed resulted in a rapid stent re-endothelialisation within hours and significant reduction of in-stent restenosis.
The results of the HEALING-II demonstrate that the EPC capturing stent technology is safe and may prevent restenosis formation in patients with normal EPC levels. In contrast, low EPC levels were associated with a late luminal loss and % volume obstruction comparable to uncoated bare metal stenting [DIRECTOR/RESTOR trial10], and TLR/TVR events. Adjudicated MACE rate was 7.9%, whereas overall clinically and non-clinically driven MACE was 19.0%. Although not powered to detect significant changes in thrombogenicity, no in-stent thrombosis was detected in the study group despite a limited time of the dual anti platelet therapy (one month). This study confirms and extends the preliminary observations of the (First-in-Man, or FIM) HEALING I study designed to establish safety and assess healing responses of this first bio-engineered stent to prevent restenosis and thrombosis in 16 patients6. The bioengineered stent used in this particular FIM study was a wet preparation stent that required pre-crimping by hand. At 6 months angiographic follow-up, a reduction of restenosis was observed using QCA and IVUS volumetric analysis (HEALING-FIM: Late luminal loss 0.63±0.21 mm, in-stent % volume obstruction 27.2±20.9%). Likewise, in-stent thrombosis did not occur in the 6 month follow-up of these patients. Ongoing development of this EPC capturing technology improved the design used now in the HEALING-II study to a dry preparation EPC stent pre-mounted on a delivery system and an improved sterilisation technique.
The concept to stimulate the vascular repair response, in order to reinstate the endogenous mechanisms to limit the exuberant proliferative response underlying restenosis, provides superior benefits as compared to the cytotoxic or cytostatic approach mediated by most drug-eluting stents (DES). These antiproliferative local drug delivery therapies however interfere with the lesion healing and foreign body reaction manifested toward the implanted stent, potentially leading to progressive neointima formation on long term follow-up11. Additionally, extensive systemic antiplatelet and antithrombotic agents are essential to prevent sub-acute and late thrombosis before the treated lesion is completely healed.
Accelerated arterial repair would have multiple beneficial effects, including improved reinstatement of the endothelial function and inhibition of neointimal formation, in addition to inhibition of platelet aggregation and thus reduced thrombogenicity of the stented vessel, improved vasomotor function, and ultimately attenuation of ongoing atherosclerosis progression. Previously, BMS implantation in the baboon AV shunt model without dual anti-platelet therapy led to thrombotic occlusion of the stented vessel within minutes, whereas stent placement of an EPC capture stent prevented in-stent thrombosis regardless of treatment with dual antiplatelet therapy14. Thus, accelerated arterial repair provides a non-thrombogenic endothelial coating of exposed stent surfaces, thereby reducing stent thrombosis and potentially decreasing or eliminating the need for anti-thrombotic therapy. Prevention of in-stent thrombosis by EPC capture mediated accelerated re-endothelialisation was confirmed by scanning electron microscopy studies of stented porcine coronaries 24-48 hours after stent placement14.
In addition, DES therapy may compromise proper vasomotor function, locally by impeded re-endothelialisation, and remote to the stented segment by interfering with endothelium-dependent vasorelaxation. Our group has previously demonstrated that the arterial segment distal to the SES-stented segment were unresponsive to acetylcholine challenge, whereas vasorelaxation upon nitrate infusion was unimpaired at 6 months following PCI, suggesting an SES-induced endothelial dysfunction in the direct vicinity of the stent, as well as at remote coronary segments1. In contrast, implantation with conventional bare metal stents did not result in an attenuated endothelium-dependent vasorelaxation response upon acetylcholine. Likewise, sirolimus stent implantation induced an exercise-induced paradoxic coronary vasoconstriction of the adjacent arterial segments suggestive of endothelial dysfunction by incomplete arterial repair2. In keeping with these previous observations, it has been recently reported that levels of EPC or CD34+ cells are highly related to vascular endothelium-dependent reactivity8, whereas preliminary studies in the pig angioplasty model demonstrated that the EPC capture technology improved the vasomotor response in the stented coronary artery as compared to sirolimus and paclitaxel eluting stent implantation15.
These studies underscore that the EPC capture technology aids, rather than disrupts, the natural repair response of the (injured) artery after intervention and thereby prevents both neointimal hyperplasia, stent thrombosis, and vasomotor function.
Flow cytometric analysis demonstrated that circulating EPC levels at 6 months follow-up directly correlate with angiographic and IVUS outcome and identified patients likely to respond to EPC capture stenting. Whereas patients with normal EPC levels appeared to respond favourably to EPC capture stenting without TLR/TVR events, patients with reduced EPC levels did not benefit from EPC capture technology and responded with significant late loss, comparable to bare metal stenting. In addition, all TLR/TVR events were restricted to this patient group with reduced EPC levels.
Various studies have shown that CAD patients reveal reduced levels and function of circulating EPCs, which correlate with CAD risk factors, including diabetes mellitus, hypercholesterolaemia, and hypertension. there be a reference here? Given the important role of EPCs for neovascularisation of ischaemic tissue, this decrease of progenitor cell levels may contribute to impaired (neo)vascularisation and vascular repair in patients with CAD. So far, no evidence has been reported regarding the role of CD34+ cells or EPCs in predicting future major cardiovascular events in patients undergoing percutaneous coronary angioplasty. The findings of a possible role of EPC levels in determining vascular response to mechanical revascularisation in humans could bear tremendous pathophysiological impacts, and will possibly provide new insights into the everyday clinical decision making. In the current study, reduced EPC levels at 6 months follow-up were associated with the absence of HMG-coA-reductase inhibitors from the pharmacotherapy of the study patients at the time of stent implantation. Moreover, statin therapy was associated with an increase of the circulating EPC titer, consistent with previous observations by the groups of Dimmeler12 and Drexler13. As EPC levels were quantified under statin therapy at 6 months, this would suggest that some of the patients do not appear to show a persistent EPC mobilisation to the systemic circulation to HMG-coA-reductase. Previously, fifteen patients with angiographically documented stable coronary artery disease, treated with atorvastatin, resulted in a 3 fold increase of EPC level by 4 weeks of treatment, with improved migratory capacity in an in vitro assay. The increase of haematopoetic progenitor cells was primarily due to an increase in CD34/KDR double positive cells, whereas the level of haematopoetic progenitor cells (CD34+/CD133+ cells) remained unchanged, suggesting accelerated differentiation towards the endothelial progenitor lineage and improved survival under statin therapy, rather than recruitment of CD34+ cells from the bone marrow. Since long term follow-up of EPC recruitment was not analysed it remains unclear whether EPC mobilisation was sustained over a longer period of time.
Study limitations
Due to the small sample size and the exploratory nature of the study, the present study did not fully evaluate the efficacy of this device. Since statin therapy has been shown to augment EPC number (and function), it may enhance the outcome of EPC capture stenting. To substantiate and extend the findings in the HEALING-FIM and the current HEALING-II clinical studies, a forthcoming HEALING-IIB clinical study will assess the safety and effectiveness of the Genous Bio-engineered R stent, in conjunction with optimal statin therapy, in the treatment of elective patients with de novo native coronary artery lesions.
Conclusions
The present study demonstrates that the EPC capture stent is safe and feasible. In patients with normal levels of EPCs, treatment with the EPC capture stent resulted in a reduction of restenosis formation after 6 month follow-up. Reduced EPCs were associated with late loss comparable to bare metal stents and TLR/TVR events, and were generally restricted to patients without statin therapy at the time of percutaneous intervention. Further study and development of this promising technology are needed to confirm the clinical efficacy of this bio-engineered stent.
Funding sources
Study supported by OrbusNeich Medical Technologies, FL, USA.

