Description
Patients with multi-vessel disease, diabetes and bifurcation lesions are among some of the most difficult lesion subgroups to treat effectively with percutaneous coronary intervention. Historically, bifurcation lesion location has been shown to be a predictor for major adverse cardiac events1. More recent studies of PCI using drug eluting stents (DES) in bifurcation lesions show that while target lesion revascularisation (TLR) rates are reduced, these lesions continue to be difficult to classify, difficult to treat, and associated with elevated complication rates such as periprocedural non-Q-wave MI2, side branch occlusion, and stent thrombosis3. These problems are not likely to be solved by procedure modifications alone; stent design modifications will play a role as well.
The procedural difficulties of treating a bifurcation lesion are related to the mismatch between the cylindrical shape of the stent and the y-shape of the bifurcation. Most techniques (T-stent, crush-stent, culotte-stent, etc.) rely on a high level of post implant device ‘fitting’ via post deployment high pressure balloon dilation in order to mould the stent into the shape of the vessel. These techniques frequently result in multiple stent overlaps, poor apposition against the vessel wall, partially obstructed vessel lumens, altered shear stress, poor expansion of the side branch stent or complete loss of access to the side branch4.
The Axxess™ stent is designed to treat the problematic anatomy at the point where the side branch diverges from the parent vessel. Shaped like a V, with the wide end oriented distally, the stent is positioned at the level of the carina and then seated within the ostia of both branch vessels (Figure 1). This shape matches the anatomy of most bifurcations <60°. Any remaining lesion – distal to the carina – can now be treated as a standard mid vessel lesion, and stent(s) can be added to one or both distal branches to complete treatment. With proper placement, the stent can be ‘deep seated’ within the lesion and span both vessels.

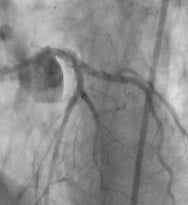
Figure 1. Left. AXXESS™ stent illustration. The self expanding stent and distal flare allows the stent to expand into the bifurcation and cover the problematic anatomy at the carina. Right. AXXESS™ stent in bifurcation, with the 3 distal markers clearly showing stent expansion in both branch vessels.
Technical specifications
The Axxess™ stent is fabricated from a nickel titanium alloy designed to operate in the super-elastic (self expanding) state. The self expansion property facilitates the seating of the stent within the ostia of the branch vessels. This provides coverage of the ostium of the side branch, and also simplifies placement of subsequent stent(s). Since the stent is symmetric, there is no need to orient the system within the vessel. The rapid-exchange delivery system allows the stent to be deployed partially (by withdrawing a covering sheath) and accurately positioned within the vessel before it is finally released.
The stent has three highly visible radiopaque markers located at the the tip of the stent to facilitate placement. These markers are also convenient for accurate placement of stents in the branch vessels. A fourth marker locates the proximal edge of the stent.
The stent is coated with the new limus class drug Biolimus A9® (Biosensors International), which is currently being evaluated for use in reducing proliferation after stenting in several trials throughout the world. The drug is delivered from the abluminal surface of the stent struts using a resorbable polymer.
The stent is provided in lengths and diameters that can accommodate vessels from 2.75 to 4.25 mm diameter. The stent is designed to exert a stable amount of force against the vessel wall over its stated diameter. The distal flare can expand to as much as 8 mm in the largest diameter stent. This is what allows the device to span large bifurcation angles. A special version of the Axxess™ stent designed for left main use is under development. This stent will span bifurcations of up to 12 mm.
History
The Axxess™ Biolimus A9 eluting stent has been under development since 2003 by Devax, Inc, a development stage company with offices in Irvine, California.
Indications for use
The Axxess™ stent is designed for the treatment of de novo bifurcation lesions where the parent vessel diameter is least 2.75 mm and the side branch is at least 2.25 mm. The angle between the branch vessels should be <60°. The Axxess™ stent is used in conjunction with other drug eluting stents such as the Cypher®.
Tip and tricks for delivery
The Axxess™ stent is particularly useful when the side branch is of significant size (>2.25 mm), and compromise of this vessel might lead to a significant infarction. Figure 2 below shows a complex bifurcation lesion treated as part of the DIVERGE trial. This left anterior descending artery (LAD) with a large proximal segment and small distal segment is particularly suitable for a self expanding stent as opposed to a T or crush technique. The branch vessels are both significantly smaller than the proximal LAD and contain diffuse disease, and require smaller but longer stents for complete coverage.
The placement technique follows. The lesion is predilated with PTCA balloons using standard bifurcation 2-wire technique. This step is necessary to create an expansion space for the Axxess™ stent. The Axxess™ stent is placed on one of the wires, preferably the wire on the more curved vessel is selected in order to facilitate the stent flare into the opposite vessel. Once deployed, the stent markers are visible as in Figure 2b. In this case, there are two in the diagonal branch and one in the LAD. The diffuse distal disease in this patient is treated with Cypher stents of appropriate length and diameter resulting in a customised, ideal fit is as shown in Figure 2c.



Figure 2. a. Large proximal vessel with diffuse disease. b. AXXESS™ Stent placed at carina. c. Final result with Cypher® stents in both branches.
Clinical experience
The Axxess™ stent has been implanted under different study protocols in over 300 patients world wide. The Axxess™ plus trial evaluated this device in a controlled multicentre trial to be published later this year of 139 patients with single de novo bifurcation lesions. This study showed that the Axxess™ procedure resulted in less than 10% composite major adverse cardiac events or MACE (death, Q- and non-Q-wave MI, TLR) at 6 months, and 7.9% restenosis rate in stented side branches.
A pilot study using a version of the Axxess™ stent designed for Left Main coronary artery bifurcation lesions has also been completed. The study enrolled 31 patients, and has completed 6 month follow-up. A clinical restenosis rate of 9% was observed, with no restenosis or late loss observed in the LMCA. Publication of the results is pending.
The Axxess™ device is currently under review for CE marking and commercial availability is expected in 2007.

