Abstract
Aims: The role of anticoagulation during percutaneous coronary intervention has been well established. However, the role of anticoagulation during diagnostic coronary angiography remains unclear. Prothrombin fragment1+2 (PF1+2) and D-dimer (DD) have been reported to be useful in evaluating thrombotic phenomena. This study was designed to determine whether activation of coagulation occurs during diagnostic coronary angiography as measured by DD and PF1+2.
Methods and results: Patients not on anticoagulation (except for aspirin) and with no documented coagulopathy undergoing elective diagnostic coronary angiography were enrolled in this prospective study. Blood samples for DD and PF1+2 were obtained serially after the femoral arterial sheath was placed. Peripheral venous blood was drawn along with an initial arterial blood sample from the sheath; thereafter, arterial blood samples from the sheath were obtained every 10 minutes for a maximum of 60 minutes or until the procedure was completed or when anticoagulation was initiated. A final venous sample was drawn at the end of the procedure. The data were analysed in time interval correlation to the DD and PF1+2 level.
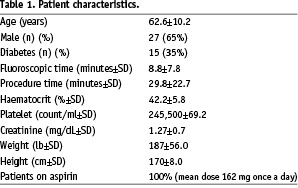
Forty-two patients were enrolled in this study, 15 were female (35%). There were 25 (59%) patients with diabetes. The mean fluoroscopic time was 8.8±7.81 minutes and the average time for the procedure was 29±22.70 minutes. There were 192 blood samples analysed. 67% of patient completed the procedure within 20 minutes and 91% within 30 minutes. Mean venous PF1+2 level was 0.20 nmol/L at baseline and 0.39 nmol/L (p=0.06) at the final interval, while the mean arterial PF1+2 level was significantly elevated. There was an increase of 0.2 nmol/L of arterial PF1+2 every 10 minutes (p<0.001). Mean venous DD at baseline and final levels were 0.41 ug/mL and 0.45 ug/mL respectively (p=0.68). There was a significant change in arterial DD with an increase of 0.02ug/ml every 10 minutes (p=0.023).
Conclusions: In diagnostic coronary angiography, there is an early rise in PF1+2 levels in blood drawn through the arterial sheath suggesting that the procedure triggers local activation of coagulation that is not observed systemically. Prophylactic anticoagulation may not be necessary in stable patients without other known risk factors who will be undergoing elective diagnostic coronary angiography for less than 30 minutes. For procedures that are prolonged, or anticipated to be prolonged greater than 30 minutes, it may be advisable to administer anticoagulation to prevent thrombus formation. These findings may not be pertinent to patients with thrombophilia.
Introduction
Coronary artery disease remains one of the major causes of mortality in the United States. Since the first cardiac catheterisation procedure performed by Forssman in 19291, coronary angiography has become an important and effective diagnostic tool in the detection of coronary arterial disease. In the early era of coronary angiography, arterial access was obtained using brachial artery cut-down, necessitating anticoagulation with intravenous heparin. A leading textbook of cardiac catheterisation stated “…the guidewire is then removed, the catheter is connected to the arterial manifold and double flushed…, followed by vigorous injection of heparinised saline solution. Full intravenous heparinisation is established immediately...”2. As the technique of coronary angiography became more efficient, this practice of administering heparin had become less common despite lack of data to support its use or its discontinuation. Thromboembolic complications during percutaneous coronary arteriography have been reported in numerous studies2. Reports of the existence of thrombotic material on the exterior percutaneously-introduced catheters in more than 50 percent of clinical studies resulting in possible arterial thromboses during catheter removal have been described3,4. Therefore, systemic heparinisation for percutaneous coronary intervention has been routinely implemented resulting in statistically fewer thromboembolic adverse events such as acute myocardial infarctions, thrombosis of the femoral artery and death2.
Thrombosis is the result of excessive procoagulant activity following the initial process of primary haemostasis. In the coagulation scheme, a series of reactions culminate in the production of sufficient thrombin to convert a small portion of plasma fibrinogen to fibrin, resulting in a network of highly cross-linked fibrin polymers, called a thrombus. D-dimer is the plasmin-mediated digestion product of Factor XIII cross-linked fibrin strands that indicates that activation of coagulation has taken place with subsequent fibrinolysis. At the more proximal end of this pathway, activated factor X cleaves prothrombin into thrombin also resulting in the formation of the activation peptide, prothrombin fragment1+2 (PF1+2)5. Elevated D-dimer levels are considered a marker of intravascular fibrin deposition, and elevated PF1+2 levels are considered a marker of activation of coagulation5.
The presence of foreign material such as a cardiac catheter or guidewire for longer than three minutes in the arterial circulation has been well documented to trigger thrombotic phenomena that could lead to devastating consequences such as cerebrovascular accident and arterial thrombosis6. It is not uncommon that diagnostic coronary angiography may be prolonged, resulting in protracted exposure of the arterial circulation to the presence of a foreign body. Therefore, we hypothesised that diagnostic coronary angiograms may also trigger activation of coagulation, particularly if the procedure lasts longer than 20 minutes. Our study was designed to evaluate whether known markers of thrombosis, D-dimer and PF1+2, are elevated during diagnostic coronary angiography. Measurements on arterial blood collected via the arterial sheath, as well as peripheral venous blood, were made to assess local (arterial sheath) versus systemic (venous system) activation of coagulation.
Methods
All patients scheduled to undergo diagnostic left coronary angiography performed through the femoral artery approach were identified and invited to participate in this prospective, observational study. Inclusion criteria involved patients equal to or greater than 18 years of age. Exclusion criteria included the following: patients who declined to participate in the study; patients who received an anticoagulant, such as unfractionated heparin, low molecular weight heparin or warfarin; patients receiving glycoprotein IIB/IIIA blocker therapy; patients with hereditary or acquired coagulation disorder(s); pregnant women, patients with acute coronary syndrome with indication for anticoagulation; and patients with severe anaemia (defined as haematocrit ≤25% within the last 48 hours). Patients on aspirin therapy were permitted to participate in the study as aspirin exerts its affect as an antiplatelet agent and will not significantly affect the coagulation cascade. For the purpose of this study, all heparin was omitted from saline flush solution. The study was approved by the Institutional Review Board at the University of Utah Health Sciences Centre.
Informed consent was obtained from eligible patients requiring diagnostic coronary angiography who had chosen to participate in this study. Citrated blood samples (3.2%) for quantitative measurement of D-dimer and PF1+2 levels were collected at the following time intervals: baseline (peripheral venous phlebotomy with 20 gauge needle) and arterial sheath sampling immediately after arterial access was achieved; and arterial sampling every 10 minutes thereafter up to one hour, or until the administration of an anticoagulant or anti-platelet agent, whichever was shorter. All arterial sampling was performed through the femoral artery via the arterial sheath (6 Fr sheath) using a plastic syringe. A 4 ml pilot sample was discarded and a 5 ml blood sample was drawn at each time point, added to the citrate tube, and mixed thoroughly. A venous sample was also collected by peripheral phlebotomy (20 gauge needle) at the same time as the collection of the final arterial sample.
D-dimer was analysed using a quantitative D-dimer assay (STA LIATEST immunoturbidimetric assay; Diagnostica Stago, Parsippany, NJ, USA), and PF1+2 was analysed using ELISA (Behring, Marburg, Germany). A D-dimer level ≥0.50 ug/ml was considered positive; while a PF1+2 level ≥1.2 nmol/L was considered positive.
Data are presented as the mean of repeated measurements of D-dimer and PF1+2 over the time course of the angiographic procedures. We utilised the student t-test for the venous samples to determine if the variation from baseline became significant, and the arterial samples were compared using a random-effects generalised least squares regression model (GLS) to test within-subject variance with time. Several arterial samples formed small clots in the citrated collection tube and were excluded from the PF1+2 data analysis.
Results
Forty-two patients were enrolled in the study (15 female and 27 male). There were 25 (59%) patients who carried a diagnosis of diabetes. Mean serum creatinine was 1.27±0.7 mg/dL, with only one patient on haemodialysis. Mean platelet count and haematocrit were 245,500/uL±69.2 and 42%±5.8 respectively. The mean fluoroscopic time was 8.8±7.8 minutes, and the average time for the procedure (from the time femoral arterial sheath inserted until post procedure haemostasis) was 29.8±22.7 minutes (Table 1).
In our analysis, 67% of patients completed the procedure within 20 minutes and 91% completed the procedure within 30 minutes procedure time. The average time of procedure reflects the efficiency of the diagnostic catheterisation procedure that is within the acceptable recommendations. There were no immediate or late complications from the procedure during immediate follow up and up to 30 days. All patients were on aspirin (mean dose 162 mg everyday). There were 192 blood samples analysed for D-dimer and 179 samples were analysed for PF1+2.
Table 2 depicts the mean and standard deviation of D-dimer and PF1+2 concentrations as correlated with time during the diagnostic procedure, determined by the described assays.
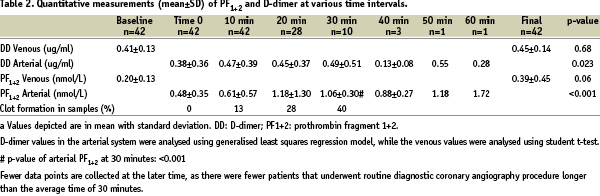
The duration of the diagnostic coronary angiography procedure performed in most patients in the study was less than 30 minutes. Therefore, few patients were available for sampling at later time periods. It was felt to be unethical to leave the patient with indwelling sheaths to complete 60 minutes blood sample collection if the procedure had been completed earlier. This is the major reason why there are few data points at the later time.
D-dimer levels at baseline from the venous circulation and at all time points collected from the arterial circulation did not differ significantly. A random-effects generalised least squares regression model to test within-subject variance with time was used to examine the statistical significance of D-dimer results from the arterial circulation. The analysis revealed that for each 10 minutes increment of the procedure, there was an increase of D-dimer by 0.02 ug/ml (p=0.023). This increase is small in comparison to patients with significant activation of the coagulation such as is seen in disseminated intravascular coagulation (DIC). The student t-test did not reveal a significant difference between baseline and final venous D-dimer level (p=0.68). With the exception of the single D-dimer measurement obtained from the arterial circulation at 50 minutes (0.55 ug/ml), all D-dimer levels were within the normal reference value of <0.5 ug/ml (Figure 1).
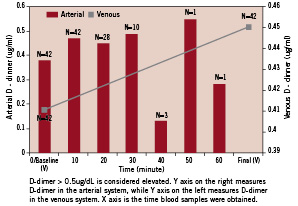
Figure 1. Arterial and venous D-dimer levels in time correlation during diagnostic angiography.
PF1+2 measurements did reveal a moderate, statistically significant transient increase in levels when measured from the arterial circulation during diagnostic left coronary angiography in the studied patients (Table 2). These values seem to plateau at 30 minutes and did not return to levels measured at baseline (Figure 2).

Figure 2. Arterial and venous PF1+2 levels in time correlation during diagnostic angiography.
Regression modelling of arterial PF1+2 levels (time 0 to 60 minutes) suggests an increase in the level of PF1+2 by 0.2 nmol/L in every 10-minute increment (p<0.001) (Table 2). One patient had a prolonged diagnostic left coronary angiography procedure lasting ~60 minutes in duration and had elevated PF1+2 levels (1.72 nmol/L) at 60 minutes with a baseline value of 0.48 nmol/L. The incidence of clot formation in the arterial specimens correlated with the length of the procedure, with 0% tubes having clots at 0 minutes, 13% at 10 minutes, 28% at 20 minutes, and 40% at 30 minutes (Table 2). No clots were formed at the longer procedure times, though the number of specimens was small. The correlation of clot formation with procedure time over the first 30 minutes suggests that the in vitro clot formation was a sign of in vivo activation of coagulation. The non-significant difference in the venous circulation between baseline and final PF1+2 levels (p=0.06; paired, student t-test) lends support to the conclusion that there may be not be significant systemic thrombotic activation.
Discussion
Activation of the coagulation cascade may occur as a result of vascular injury, low level stimuli such as immune system activation of coagulation, viral or bacterial infections, or as a response to tissue factor expression. Thrombin is a very unstable and easily degradable substance that cannot be easily measured directly7. Therefore, indirect assays are employed to monitor activation of coagulation by measuring the levels of specific markers of coagulation activation and clot lysis, including PF1+2 and D-dimer. PF1+2 formation occurs early in the coagulation cascade, while D-dimer generation occurs after clot formation, crosslinking, and fibrinolysis.
Quantitative assays for D-dimer are very sensitive for detecting the presence of thrombosis, exhibiting high negative predictive value8. Numerous studies have provided evidence for the correlative value of D-dimer in thrombus formation9. The absolute increase of D-dimer levels not only correlates with a thrombotic event, but its detection is also sufficiently sensitive to correlate with severity of atherosclerosis12. D-dimer and PF1+2 have significant sensitivity and high negative predictive value in evaluating the activation of coagulation and thrombotic phenomena10. Elevated D-dimer and PF1+2 levels have been reported in various thrombotic conditions, particularly in patients with deep vein thrombosis, pulmonary embolism and arterial thromboembolism8,11. However, conflicting evidence exists regarding the correlative value of D-dimer and PF1+2 in coronary thrombosis. Barakett12 and colleagues detected significantly higher D-dimer levels in a prospective study of 12 ACS patients with acute coronary syndrome without ST elevation that correlated positively with the severity and complexity of coronary lesions. They did not measure PF1+2 levels. Panchenko and colleagues reported that patients with the most extensive atherosclerosis had the highest D-dimer levels that correlated with reported clinical symptoms and other diagnostic tools including coronary angiography and ultrasound Doppler9. Giannitsis and colleagues13 did not detect any elevation in D-dimer levels, but did detect elevated levels of PF1+2 that correlated positively with severity of angiographically-verified coronary artery disease.
PF1+2 is a by-product of thrombin formation from prothrombin and therefore serves as an indirect marker of thrombin formation. The value of PF1+2 in detecting prothrombin activation was reported by Teitel10. Serum PF1+2 concentrations exceed normal reference levels in severe hypercoagulable states14,15.
Selective coronary angiography is a commonly performed procedure, with a small risk of cerebrovascular accident and peripheral thrombosis. It has been well documented that percutaneous coronary intervention may trigger a high risk of thrombotic events if IV heparin anticoagulation is not utilised. Nevertheless, the role of systemic anticoagulation therapy during diagnostic coronary intervention has not been clearly elucidated.
Our study evaluated the hypothesis that diagnostic coronary angiography may also trigger activation of the coagulation cascade as measured by the elevation of known markers of activation such as D-dimer and PF1+2. The results suggest that diagnostic coronary angiography triggers activation of the coagulation cascade locally in the sheath as determined by the statistically significant elevation of PF1+2 and D-dimer level in the arterial system from baseline, and immediately at the completion of the procedure from the venous circulation (p ≤ 0.001) (Table 2). However, venous D-dimer and PF1+2 are not significantly changed (p=0.68). In fact, D-dimer levels continued within the normal reference range of <0.5 ug/ml in all but one patient who had a prolonged diagnostic coronary angiogram for 50 minutes in duration. This sole sample and the absence of an upward trend in D-dimer concentrations from 50 to 60 minutes, supports the assumption that this may be due to the prolonged procedure or an outlier value. In contrast to the fairly flat trend of D-dimer concentrations as the duration of diagnostic coronary angiograms is prolonged, PF1+2 levels showed a trend toward elevation in patients whose procedure lasted for 10 minutes (from 0.61 nmol/L to 1.18 nmol/L) or longer. This trend toward increased PF1+2 levels in the arterial sheath samples reached statistical significance. The direct correlation of clot formation with procedure time suggests that the in vitro clot formation was a sign of in vivo activation of coagulation. In addition, there was no immediate or late complications (30 days) due to thromboembolic event during follow-up.
Jung and colleagues16 observed similar results when the non-ionic contrast agent, iopromide was utilised during the diagnostic cardiac catheterisation procedure. PF1+2 levels were found to be elevated at 30 minutes following the completion of the procedure when compared to baseline, while D-dimer levels did not change. The authors did not specify the average duration of the procedure. These findings were in contrast to those demonstrated by Biancardi and colleagues17 who found that PF1+2 levels were not statistically different before and after coronary angiography, regardless of the type of contrast media used. In both of these described studies, only pre- and post-treatment levels (the exact duration is not indicated) of PF1+2 were measured. To date, we are not aware of any publication where coagulation markers, including D-dimer and PF1+2 were measured at any interval during diagnostic coronary angiograms or percutaneous coronary interventions. Namiki and colleagues7 measured various haemostatic markers including PF1+2 under various procedures including diagnostic coronary angiography. In this small population of patients, they did not detect any changes in PF1+2 levels at 24 hours and three days after the procedure was completed. Once again, the level of PF1+2 was not measured during the duration of the procedure.
Our results indicate proximal activation of the coagulation cascade; however, the quantitative level of D-dimer did not change. One possibility is that inhibitors to activated coagulation factors may have exerted their effects to inhibit thrombus formation and the formation of D-dimer. We suggest that the non-significant elevation of D-dimer is probably due the activity of natural anticoagulants, such as protein C, protein S, and antithrombin.
The principal limitation in our study is the limited number of patients enrolled in this prospective, observational study. This may have limited our ability to detect small changes in D-dimer. However, our results tend to exclude clinically important increases in D-dimer.
Administration of anticoagulation for all diagnostic coronary angiography is being practice by some operators. Whilst based on our study, it is recommended for prolonged procedure or anticipated prolonged procedure, the risk and benefit of preventing thrombus and bleeding should be considered if anticoagulation is to be administered in all patients, considering the risk of thrombus formation within 30 minutes is small. The same consideration should be taken into mind when recommending the administration of clopidogrel with aspirin for diagnostic coronary angiography. In addition, the presence of clopidogrel may delay surgery without much benefit in otherwise stable patients who need urgent surgical procedures. Certainly, unfractionated heparin is a better choice for anticoagulation as it has a shorter half-life and can be quickly reversed with protamine.
A randomised trial to clarify the role of anticoagulation in preventing thrombus formation during routine diagnostic coronary angiography in a randomised control study would require too many patients to be practical. However, a study to evaluate the impact of anticoagulation (i.e., heparin) for arterial access on bleeding during diagnostic coronary angiography would be feasible.
We conclude that prophylactic anticoagulation may not be necessary in stable patients without known risk factors undergoing elective procedure lasting less than 30 minutes; however, these findings may not be pertinent to patients with thrombophilia. In an anticipated prolonged procedure more than 30 minutes, it may be advisable to administer anticoagulation to prevent thrombus formation.
Acknowledgements
The authors would like to sincerely express gratitude to Camille Dominguez, MLT, ASCP, Joshua Holdstock, RN; Marie Magnus, RN; Sally Haub, RN; Annette Korsell, RN; Tracy May, RN for their invaluable technical assistance in making the study possible and efficient. The authors would like to specially thank Dr. Nathan Segerson for his invaluable assistance in statistical analysis.

