Description
Abbott Vascular’s side branch access everolimus eluting coronary stent system (SBAEECSS) stent leverages technology of the company’s XIENCE V DES stent with everolimus drug coating and similar scaffolding. The device is designed for side branch preservation utilising a provisional T stenting approach, an approach that gives physicians the option of postponing the decision to stent the side branch until after the main branch is stented.
History
Treatment of bifurcation disease is consistently reported by interventional cardiologists as one of the major clinical challenges. Traditionally, treatment of bifurcation disease is described as more difficult, with poorer long term outcomes compared to treatment of workhorse lesions. Furthermore, there is a multitude of techniques with no agreed upon standard. Recent studies, such as the Nordic Bifurcation study (Erglis, Andrejs. “Eight Months Angiographic Follow Up in Patient Randomised to Simple or Complex Stenting of Coronary Artery Bifurcation Lesions.” TCT Conference 2006), have suggested that the provisional T method is suitable for routine non-complex bifurcation stenting. Yet, using standard drug eluting workhorse stents for this method is not always ideal, as it involves manipulation of the stent in a manner in which it has not been designed or indicated. A stent cell can be distorted too wide or not enough, leading to dissection or stent flaring. Therefore, the clear physician need is for a simple, predictable provisional T technique that is suitable for the majority of bifurcation disease, with long term results for both main and side branches comparable to those seen in workhorse lesions.
Technical specifications
A side branch access (SBA) stent is crimped onto two balloons. The main branch balloon, which is mounted on a rapid exchange lumen, extends the length of the stent. The side branch balloon, which is mounted on an over-the-wire (OTW) lumen, exits the stent at the mid-point. Proximal to these two balloons, the two inflation lumens are joined into a single common inflation lumen that can be pressurised with a single inflation device. The OTW lumen is occupied with a wire mandrel that exits the side branch balloon tip and immediately enters a pocket along side the extended main branch balloon tip, effectively joining the two balloon tips together. The deployment sequence of the device is illustrated in Figures 1-5.
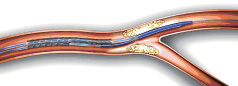
1. Advance system into the main branch, over conventional RX wire.

2. Retract joining mandrel to release the OTW side branch tip. Insert exchange length guide wire.
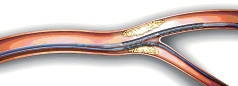
3. Position guide wire in the side branch and advance system to the carina.
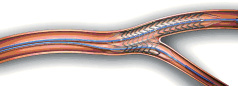
4. With a single inflation device, stent is deployed with a single simultaneous inflation.

5. Following deflation, the delivery system is retracted, preserving access to both branches.
Figures 1-5. Deployment sequence.
The Abbott Vascular SBA everolimus eluting system includes a unique joining mandrel, which holds the main branch and side branch tip together helping to avoid wire wrap – a common occurrence when sending two guide wires through the catheter. The device is advanced over a single wire until it reaches the target site, where the joining mandrel releases the side branch guide wire. This subsequently allows the device to rotate into proper phase at the side branch’s ostium. Also the device’s tips and shaft materials were engineered to facilitate tracking over the guide wires and the guided rotation into phase, adding to the ease of use and a predictable procedure. Finally, the stent is deployed with simultaneous kissing balloon inflation, with one inflation device to minimise plaque shift, allowing deployment of the stent safely and quickly.
Stent characteristics
Deployment: balloon expandable
Alloy: cobalt chromium
Strut thickness: 0.0034 inches
Angles: 75° (maximum)
Delivery System
Guide compatibility: 6 French
Tracking: dual wire
Delivery balloon: straight
Marking system: 3 balloon shaft markers
Stent markers
There are three markers in total. There are two shaft markers delineating the proximal & distal extent of the main branch stent, and one shaft marker delineating the carina of the side branch.
Indications for use
The Abbott Vascular everolimus eluting SBA stent is currently in development and not yet available for sale. The product’s suggested indication will be for improving coronary luminal diameter in patients with symptomatic ischaemic heart disease due to discrete de novo or restenotic native coronary arteries lesions (length <15 mm) with a reference vessel diameter of 2.5 mm involving a major side branch with a diameter >2.0 mm, or with a reference vessel diameter of 3.0 to 4.0 mm involving a major side branch with a diameter >2.5 mm.
Tips and tricks for delivery (use)
Following wiring and pre-dilatation of both branches, the main branch RX balloon tip is back-loaded onto the wire in the main branch. With the two balloon tips joined by the mandrel wire, the system is advanced to a point just proximal to the target bifurcation. The joining mandrel wire is then removed by unlocking it at the proximal adapter hub, and withdrawing it from the OTW lumen. A new wire is then introduced into the OTW lumen to exit the side branch balloon tip, and placed in the side branch vessel. The system is then advanced into the bifurcation until forward motion stops. With a single inflation device, both the main and side branch balloons are pressurised, deploying the stent in the main branch and opening a portal into the side branch (Figure 6).
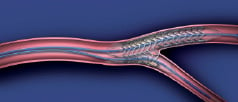
Figure 6. Schematic representing the special relationship between a deployed Abbott Vascular everolimus eluting side branch access stent and the bifurcation.
Preclinical experience
A porcine coronary acute performance study was conducted to evaluate the ease of deployment and stent placement of the side branch access everolimus eluting coronary stent system (SBAEECSS). Stents were deployed into either the left anterior descending coronary artery (LAD) and affected diagonals, or the left circumflex coronary artery with the appropriate marginals of four swine. The stents were deployed with a target balloon to artery ratio of 1.1 to 1.0. Following the procedure the animals were humanely euthanised and the hearts were pressure perfusion fixed. Radiographs were obtained to locate and assess stent placement in the coronary vasculature. Subsequently, the stents were processed for histology and longitudinal vessel segments were cut and stained with hematoxylin and eosin (H&E) to evaluate stent placement and strut apposition at the main and side branches.
The stents were well placed at the bifurcation carina with even expansion of the stent in the main branch and preservation of the side branch lumen. Histologic analysis demonstrated that the stent struts were well apposed to the vessel wall in the main branch, with minimal acute injury to the vessel wall. The side branch was patent with good mechanical support of the carina, and minimal disruption to the side branch anatomy. These features should cause minimal flow disruption, thereby resulting in reduced risk for thrombosis as well as subsequent stenosis of the side branch (Figure 7).

Figure 7. Longitudinal section, H&E 5 x (B)
Future direction
Currently, the Company is conducting preclinical studies using porcine and ovine coronary models. These studies will evaluate the acute performance and the safety of the system in order to begin human clinical trials. The side branch access everolimus eluting coronary stent system (SBAEECSS) will offer a safe and dedicated provisional T stenting approach to physicians, allowing them to easily and predictably treat bifurcation lesions.

