Tricuspid regurgitation is associated with increased morbidity and mortality, thus warranting devices for appropriate therapy.
An 81-year-old male with torrential functional tricuspid regurgitation (TR; biplane vena contracta [VC] width 30 mm, effective regurgitant orifice area [EROA] 2.04 cm²) (Figure 1A-Figure 1B-Figure 1C, Moving image 1-Moving image 2-Moving image 3) suffered from recurrent episodes of congestive heart failure despite optimal medical therapy, including an extensive diuretic regimen. Given the advanced stage of his disease, with severe dilatation of the tricuspid annulus (diameter 72 mm, gap 18 mm) and inferior vena cava (IVC; diameter 65 mm), none of the currently available devices were deemed suitable. Other echocardiographic findings showed preserved left and borderline right ventricular (RV) function (three-dimensional [3D] right ventricular ejection fraction 44%, tricuspid annular plane systolic excursion 17 mm, fractional area change 33%), yet an impaired RV stroke volume index (SVi; 30 mL/m²) (Figure 1B). Right-heart catheterisation excluded any form of pulmonary hypertension. Medical history included permanent atrial fibrillation and previously conducted coronary artery bypass graft surgery, as well as mitral transcatheter edge-to-edge repair. Since screening for all commercially available devices (including transcatheter annuloplasty, transcatheter edge-to-edge repair and transcatheter valve replacement) remained unsuccessful, application for off-label utilisation (compassionate use) of a novel transcutaneously implanted spacer (Pivot Extend [Tau Medical]) was permitted by national authorities.
The procedure was conducted under general anaesthesia with transoesophageal echocardiographic guidance. As the first step, a stiff wire was placed in the right lower pulmonary artery branch by successive exchanges via right femoral vein access. Subsequently, the position of the wire was assessed in the right ventricle to exclude entanglement and/or interference with the tricuspid subvalvular apparatus and papillary muscles (Moving image 4, Moving image 5). The device was then brought into position (Moving image 6), and its body was inflated to a diameter of 12 mm, which resulted in approximately 50% reduction of TR (Figure 1B, Figure 1D, Figure 1E). Finally, the spiral anchor was deployed into the IVC without any notable complications. Besides macrohaematuria due to urinary catheter placement, postprocedural observation remained uneventful. Discharge echocardiography showed massive TR (VC width 16 mm, EROA 0.90 cm²) with an improved RV SVi of 45 mL/m², which was also confirmed at ambulatory follow-up (Figure 1B, Figure 1F, Moving image 7, Moving image 8).
The Pivot Extend marks the comeback of spacer technology for the treatment of TR and represents a potential all-comer device, irrespective of transvalvular cardiac implantable electronic device leads, periprocedural imaging quality, and anatomical limitations. The previously reported features were also observed during the first-in-human application. Its atraumatic anchoring system, consisting of the elephant trunk and the spiral anchor, led to device fixation in the anterior-posterior axis, aiming to eliminate the risk for structural damages, including perforation, which was an Achilles’ heel of previous spacer devices. On the other hand, the device was still able to move in the septal-lateral axis, causing a self-centring effect by following physiological valve movement. Thus, it was always positioning centrally, directly filling the gap, and adequately reducing TR by enhancing coaptation. Another key feature is the vertical passing of the 3D leaflet, also aiming to improve therapeutic efficacy12. Further clinical data are required to assess the efficacy and safety of this novel technology.
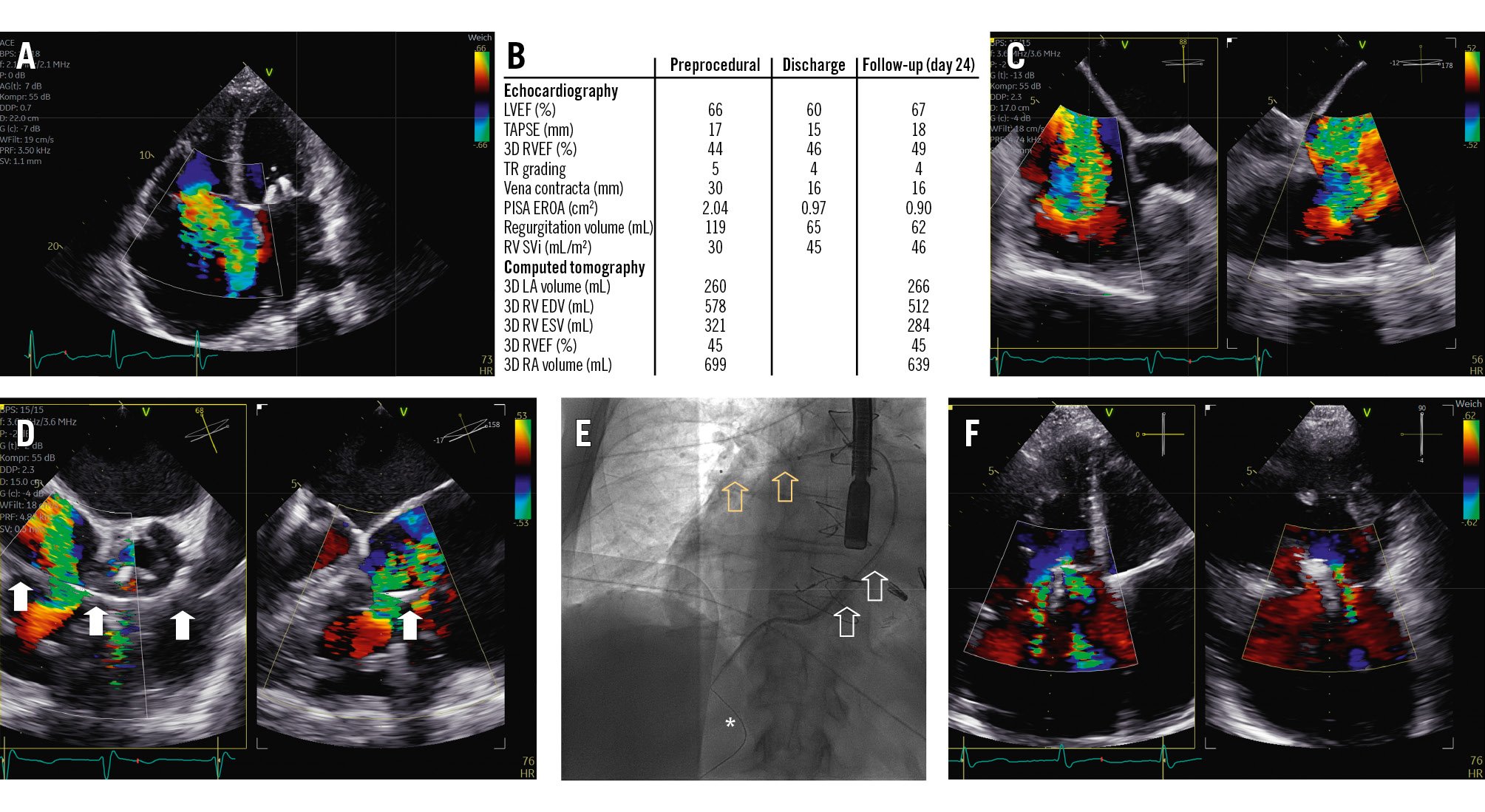
Figure 1. Overview of periprocedural imaging and procedural results. A) Torrential TR severity. B) Measurements by transthoracic echocardiography and computed tomography at baseline, discharge and follow-up. C) Intraprocedural transoesophageal echocardiography confirming TR severity. D) The spacer (arrows) already in position with reduction of TR by approximately 50%. E) Final positioning of the device (white arrows) with its respective spiral anchor (asterisk) and elephant trunk (yellow arrows). F) Residual TR at follow-up. 3D: three-dimensional; EDV: end-diastolic volume; ESV: end-systolic volume; EROA: effective regurgitant orifice area; LA: left atrial; LVEF: left ventricular ejection fraction; PISA: proximal isovelocity surface area; RA: right atrial; RV: right ventricular; RVEF: right ventricular ejection fraction; SVi: stroke volume index; TAPSE: tricuspid annular plane systolic excursion; TR: tricuspid regurgitation
Acknowledgements
Ethical approval was waived as the device was utilised as compassionate use; the application for intended use was examined and accepted by the “Bundesinstitut für Arzneimittel und Medizinprodukte”.
Conflict of interest statement
F. Barbieri reports grant support from Abbott and Boston Scientific; has received consulting fees from Boston Scientific and Edwards Lifesciences; and has received speaker honoraria from Edwards Lifesciences. The other authors have no conflicts of interest relevant to the contents of this paper to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Baseline transthoracic apical four-chamber view displaying borderline preserved right ventricular ejection fraction and a severe gap.
Moving image 2. Baseline transthoracic subxiphoid long-axis view displaying severe dilatation of the inferior vena cava.
Moving image 3. Intraprocedural transoesophageal biplane short-axis view to demonstrate dimensions of the annulus, leaflet length, and gap size.
Moving image 4. Intraprocedural transoesophageal transgastric long-axis view demonstrating wrong wire positioning, entangled in the subvalvular apparatus.
Moving image 5. Intraprocedural transoesophageal transgastric long-axis view demonstrating correct wire positioning.
Moving image 6. Intraprocedural transoesophageal 3D en face view displaying the self-centring effect of the device. While being fixated in the anterior to posterior axis, the device is able to move from medial to lateral, allowing it to passively position itself in the middle of the valve.
Moving image 7. Postprocedural transthoracic parasternal short-axis view showing correct positioning of the device with its respective self-centring effect at follow-up.
Moving image 8. Postprocedural coronal computed tomography displaying the positioning of the device.

