Abstract
Despite widespread adoption of acetylsalicylic acid and P2Y12 receptor inhibitor therapy as the standard of care for secondary event prevention in patients with acute coronary syndrome (ACS), the rate of cardiovascular death or myocardial infarction following discharge is approximately 24-31% over five years, indicating an important unmet need to reduce further the risk of recurrent ACS events. Because thrombin has a role in arterial thrombus generation, a mechanistic rationale exists for adding an anticoagulant to dual antiplatelet therapy to reduce cardiovascular event rates and mortality. The direct thrombin inhibitor dabigatran and the direct Factor Xa inhibitors rivaroxaban and apixaban have been investigated for this application, with only rivaroxaban successfully completing a phase III trial. These results suggest that dose selection is of paramount importance in this indication, with lower anticoagulant doses (relative to those used in other indications, such as stroke prevention in atrial fibrillation) plus low-dose acetylsalicylic acid potentially improving cardiovascular outcomes. This article reviews clinical trial data of anticoagulants for secondary event prevention in patients with ACS; it also discusses the mechanistic reasons that may underlie these observations and looks towards the potential impact of findings from the ATLAS ACS 2 TIMI 51 trial on clinical practice.
Abbreviations
APPRAISE: Apixaban for Prevention of Acute Ischemic Events
APRICOT-2: Antithrombotics in the Prevention of Reocclusion In Coronary Thrombolysis-2
ARISTOTLE: Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation
ASPECT-2: Anticoagulants in the Secondary Prevention of Events in Coronary Thrombosis-2
ATLAS ACS: Anti-Xa Therapy to Lower cardiovascular events in Addition to aspirin with or without thienopyridine therapy in Subjects with Acute Coronary Syndrome
AVERROES: Apixaban Versus Acetylsalicylic Acid to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment
CARS: Coumadin Aspirin Reinfarction Study
CHAMP: Combination Hemotherapy And Mortality Prevention Study
CURE: Clopidogrel in Unstable angina to prevent Recurrent Events
ESTEEM: Efficacy and Safety of The oral dirEct thrombin inhibitor ximelagatran in patients with recEnt Myocardial damage
GUSTO: Global Use of Strategies To Open Occluded Coronary Arteries
PENTUA: Pentasaccharide in Unstable Angina study
RE-DEEM: Randomised Dabigatran Etexilate Dose-Finding Study In Patients With Acute Coronary Syndromes Post Index Event With Additional Risk Factors For Cardiovascular Complications Also Receiving Aspirin and Clopidogrel
RUBY-1: Safety, Tolerability and Efficacy of Darexaban (YM150) in Patients with Acute Coronary Syndrome: a Phase II Study
TIMI: Thrombolysis In Myocardial Infarction
WARIS-2: The second Warfarin-Aspirin Re-Infarction Study
Introduction
Acute coronary syndrome (ACS) includes: unstable angina or myocardial infarction (MI), either ST-segment elevation (STEMI) or non-ST-segment elevation (NSTEMI) determined by electrocardiogram. All three share a common pathophysiology: acute intracoronary thrombus formation on disrupted atheromatous plaques. Antithrombotic therapy is recommended for the long-term prevention of further ischaemic events. Although monotherapy with acetylsalicylic acid (ASA) has been shown to reduce the occurrence of the combination of MI, stroke and vascular death, and also death from any cause1, the CURE study2, which evaluated ASA plus the P2Y12-receptor antagonist clopidogrel in patients with NSTEMI-ACS, demonstrated a 20% reduction in the risk of cardiovascular death, MI or stroke compared with ASA alone (relative risk 0.80; 95% confidence interval [CI]: 0.72-0.90; p<0.001). The benefits of dual antiplatelet therapy (DAPT) for ischaemic endpoint prevention after ACS were evident after the first month and for up to one year. DAPT is, therefore, the current mainstay thromboprophylaxis in patients who experience ACS3-6. However, despite high usage of secondary prevention strategies, the five-year post-discharge rate of vascular death or MI after ACS is still approximately 24%, 31% and 27% for patients with unstable angina, NSTEMI and STEMI, respectively7.
The success of DAPT is attributed to the belief that thrombus formation in ACS is predominantly platelet-driven. However, arterial plaque rupture also triggers coagulation cascade activation, leading to thrombin generation and, ultimately, a fibrin-containing thrombus (Figure 1). Disrupted arterial plaques exhibit persistent plaque instability8,9 consistent with observations of long-term elevated thrombin generation after an acute ACS episode10,11. These observations led to the development of the “thrombin hypothesis”, which proposed that better clinical outcomes for patients with ACS could be achieved through greater thrombin inhibition12,13. However, a series of trials with both broad-acting and more selective anticoagulants conducted over the past decade have mostly failed to validate the thrombin hypothesis14.
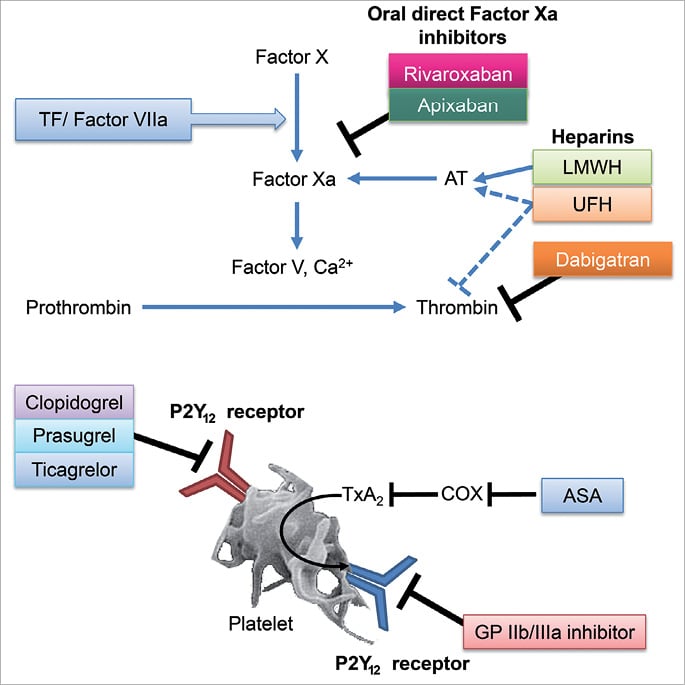
Figure 1. Mechanism of action of anticoagulants and antiplatelets. Factor Xa and Factor Va bind to each other on the phospholipid membranes of platelets to form the prothrombinase complex, which enzymatically converts prothrombin to thrombin. Thrombin then cleaves fibrinogen to fibrin, resulting in clot formation. Adapted from Antman 200151. ASA: acetylsalicylic acid; AT: antithrombin; COX: cyclooxygenase; GP: glycoprotein; LMWH: low molecular weight heparin; TF: tissue factor; TxA2: thromboxane A2; UFH: unfractionated heparin
Dosing and mechanisms of action can have important consequences for a given therapy. Historically, oral anticoagulants were not widely used in the ACS setting owing to lack of efficacy or bleeding concerns; however, data from the recent ATLAS ACS 2 TIMI 51 study suggest that patients with ACS receiving standard antiplatelet therapy (low-dose ASA alone or ASA plus clopidogrel or ticlopidine) may benefit from the addition of low-dose rivaroxaban. The implications of the ATLAS ACS 2 TIMI 51 findings for clinical practice could be quite substantial.
In addition to discussing the rationale for a “dual pathway” strategy (i.e., anticoagulant and antiplatelet therapy) in patients with ACS, this review summarises clinical trial findings for anticoagulants in the ACS setting and discusses the clinical implications of the results of the ATLAS ACS 2 TIMI 51 study.
Anticoagulants in acute coronary syndrome
PARENTERAL ANTICOAGULANTS
Heparin has long been used as short-term treatment in patients with ACS. However, it is relatively ineffective at inhibiting clot-bound thrombin15. The idea that a more “targeted” anticoagulant such as a direct thrombin inhibitor might improve patient outcomes (in line with the thrombin hypothesis) was tested in the GUSTO IIb and TIMI 9b trials16,17. The former study demonstrated that, compared with heparin, a parenteral direct thrombin inhibitor (hirudin) significantly reduced the combined primary endpoint of mortality and non-fatal MI during the first two days after infusion (2.3% vs. 3.1%; odds ratio 0.73; 95% CI: 0.59-0.91; p=0.001); however, this significance was lost at 30 days (p=0.06)16. Similarly, results from TIMI 9b did not demonstrate a significant reduction in mortality and MI with hirudin at 30 days17. Commentators at the time suggested possible reasons for this (e.g., similar effectiveness at selected doses; no additional benefit on top of thrombolytic therapy and ASA), concluding that the thrombin hypothesis had neither been proven nor disproven12,13. Heparin and hirudin are both associated with rebound hypercoagulability after their discontinuation18,19. Furthermore, longer-duration anticoagulation is necessary to address the issue of persistent thrombin generation in patients with ACS, a strategy that renders parenteral anticoagulation impractical.
VITAMIN K ANTAGONISTS
Oral vitamin K antagonists (VKAs), such as warfarin, have been the only oral anticoagulants available for use in the ACS setting. VKAs inhibit the synthesis of several coagulation cascade components, including protein C and protein S20. Data from the CARS21 and CHAMP22 trials showed that low-intensity warfarin (international normalised ratio [INR] <2 and 1.5-2.5 for CARS and CHAMP, respectively), when administered in combination with low-dose ASA in patients post-ACS, provided no additional clinical benefit over ASA alone. In contrast to these findings, outcomes from the APRICOT-223, ASPECT-224 and WARIS-225 trials showed that, when the intensity of anticoagulation was increased (INR 2.0-3.0 for APRICOT-2 and ASPECT-2; INR 2.8-4.2 for WARIS-2), VKAs in combination with low-dose ASA produced greater therapeutic benefits than ASA monotherapy26. However, these benefits were generally attenuated by an increased risk of bleeding – findings that were corroborated in two meta-analyses27,28. Notably, warfarin did not benefit mortality in either meta-analysis.
Based on their mechanism of action, all antithrombotics have the potential for serious bleeding events. VKAs also have other substantial limitations: for example, multiple drug and food interactions result in unpredictable anticoagulation that necessitates routine coagulation monitoring. These limitations spurred the development of a “new generation” of oral anticoagulants.
XIMELAGATRAN
Ximelagatran was the first of this new generation to emerge, capable of inhibiting both free and clot-bound thrombin and offering potential advantages over heparins and VKAs. The phase II ESTEEM study evaluated the efficacy and safety of ximelagatran for the long-term treatment of patients with ACS, with or without ST-segment elevation29. Patients were randomised to ximelagatran in combination with ASA or to ASA alone. The four combined doses of ximelagatran assessed were shown to reduce significantly the incidence of the primary composite endpoint of death, MI and severe recurrent ischaemia by 24% compared with placebo (16.3% vs. 12.7%; hazard ratio [HR] 0.76; 95% CI: 0.59-0.98; p=0.036) over the course of the sixmonth study period. There was no significant difference in major bleeding event rates between the ximelagatran and placebo treatment arms (1.8% vs. 0.9%). Mortality was low, averaging 3% in all groups. Data from an ESTEEM substudy further showed that ximelagatran induced a prolonged and stable reduction in thrombin generation30. Although ximelagatran was later withdrawn from the market because of liver toxicity concerns, ESTEEM provided initial support for the concept of long-term treatment with a novel oral anticoagulant for secondary prevention in ACS.
DABIGATRAN
Dabigatran, a direct thrombin inhibitor, reversibly inhibits both free and clot-bound thrombin (Figure 1). RE-DEEM was a phase II, dose-ranging study31, in which 1,861 patients with NSTEMI or STEMI ACS were randomised to receive one of four doses of dabigatran or placebo. Ninety-nine per cent of patients were already receiving DAPT. Dose-dependent increases in the primary composite endpoint of major bleeding or clinically relevant minor bleeding events were found for dabigatran (3.5%, 4.3%, 7.9% and 7.8% in the 50 mg, 75 mg, 110 mg and 150 mg groups, respectively) compared with 2.2% in the placebo group.
All dabigatran doses consistently and significantly reduced D-dimer levels, indicating a decrease in thrombin generation, which was also associated with lower cardiovascular event rates31. However, low event rate and lack of power for efficacy rendered the net clinical benefit inconclusive. At the time of writing, no plans for a phase III trial with dabigatran have been disclosed.
APIXABAN
Factor Xa occupies a pivotal position upstream of the final common pathway of coagulation. Factor Xa inhibition should theoretically provide a more efficient mechanism for the control of thrombus formation. Unlike direct thrombin inhibitors, inhibition of Factor Xa inhibits thrombin generation without interfering with the enzymatic activity of existing thrombin. Factor Xa inhibitors, therefore, do not block thrombin-mediated protein C activation, potentially providing anticoagulation without adversely affecting haemostasis.
Apixaban is an oral, direct Factor Xa inhibitor that inhibits both free and clot-bound Factor Xa (Figure 1), and it has been evaluated for use in ACS in the phase II APPRAISE32 and phase III APPRAISE-233 studies.
APPRAISE was a dose-ranging study in which subjects (n=1,715) with STEMI, NSTEMI or unstable angina were randomised to either a 2.5 mg twice daily (bid), 10 mg once daily (od), 10 mg bid or 20 mg od dose of apixaban (or placebo), in tandem with antiplatelet monotherapy or DAPT32. A numerical trend towards a lower incidence of ischaemic events in patients receiving apixaban compared with placebo was reported. However, dose-dependent increases in bleeding occurred with unacceptable bleeding rates for the two highest doses of apixaban. This prompted the Data Monitoring Committee to recommend discontinuation of these study arms. However, these phase II findings were sufficient to warrant further study at phase III.
APPRAISE-2 (Table 1) enrolled 7,392 patients with ACS who were at high risk of recurrent ischaemic events. Although a 5 mg bid dose of apixaban was not evaluated in the phase II APPRAISE study, patients in APPRAISE-2 were randomised to receive either apixaban 5 mg bid or placebo in combination with standard antiplatelet therapy33. Ninety-seven per cent of patients received ASA, with 81% receiving DAPT. After a median follow-up of 241 days, 7.5% of patients assigned to apixaban had a composite endpoint event of cardiovascular death, MI or ischaemic stroke (13.2 events per 100 patient-years), compared with 7.9% of patients receiving placebo (14.0 events per 100 patient-years; HR 0.95; 95% CI: 0.80-1.11). TIMI major bleeding (Thrombolysis In Myocardial Infarction definition) occurred in 1.3% of patients who received ≥1 dose of apixaban (2.4 events per 100 patient-years) compared with 0.5% of patients in the placebo arm (0.9 events per 100 patient-years) (HR 2.59; 95% CI: 1.50-4.46; p=0.001). Compared with placebo, patients in the apixaban group had more intracranial bleeding (0.6 vs. 0.2 events per 100 patient-years; HR 4.06; 95% CI: 1.15-14.38; p=0.03). Fatal bleeding occurred in five patients taking apixaban compared with none in the placebo arm. Because the increase in bleeding events with apixaban compared with placebo was not offset by a decrease in ischaemic events, APPRAISE-2 was discontinued prematurely. These results may have been caused by the selection of too high a dose of apixaban for use in patients with ACS: this is discussed later.
DAREXABAN
Darexaban (YM150) is a direct Factor Xa inhibitor, the clinical development programme for which has now been discontinued34. Darexaban was evaluated in patients with ACS in the RUBY-1 trial35. In this phase II, dose-finding study, patients with recent high-risk NSTEMI or STEMI (n=1,279) were given darexaban (5 mg bid, 10 mg od, 15 mg bid, 30 mg od, 30 mg bid or 60 mg od) or placebo over 26 weeks in addition to standard antiplatelet therapy. Although there was a dose-dependent increase (p=0.009) in major or non-major clinically relevant bleeding with darexaban (with HRs ranging from 1.8 to 3.8 vs. placebo), there was no decrease in the composite rate of death, stroke, MI, systemic thromboembolic events and severe recurrent ischaemia with darexaban. However, RUBY-1 was underpowered to assess efficacy.
RIVAROXABAN
Rivaroxaban is also an oral, direct Factor Xa inhibitor that inhibits both free and clot-bound Factor Xa (Figure 1), and has been evaluated for the prevention of recurrent ischaemic events in patients with ACS. The phase II ATLAS ACS TIMI 46 trial evaluated four doses of rivaroxaban (total daily doses of 5 mg, 10 mg, 15 mg and 20 mg given either once daily or divided into bid regimens) in a patient population (n=3,491) almost double the size of that studied in either RE-DEEM or APPRAISE36. Over a six-month study period, rivaroxaban in combination with either antiplatelet monotherapy or DAPT reduced the primary efficacy endpoint (death, MI, stroke or severe recurrent ischaemia requiring revascularisation) compared with antiplatelet monotherapy or DAPT alone, although the efficacy benefits occurred at the expense of an increase in the risk of bleeding. The two lower doses (2.5 mg and 5 mg bid) were subsequently carried forward to the phase III ATLAS ACS 2 TIMI 51 trial based on favourable benefit–risk profiles.
In the phase III ATLAS ACS 2 TIMI 51 trial (Table 1), more than 15,000 patients with all types of ACS received either ASA monotherapy or DAPT with ASA plus a thienopyridine (the latter arm comprising 93% of patients), based on the investigator’s decision. Patients receiving a thienopyridine were given either clopidogrel or ticlopidine. Prasugrel or ticagrelor use was not permitted because these agents were either unapproved or commercially unavailable at the time of the trial. Patients were randomised to receive rivaroxaban (2.5 mg or 5 mg bid) or placebo. Patients were enrolled within seven days of hospital admission for the index event and needed to be stabilised before enrolment37.

Overall, rivaroxaban significantly reduced the primary event rate (the composite of cardiovascular death, MI or stroke) compared with placebo (8.9% vs. 10.7%; HR 0.84; 95% CI: 0.74-0.96; p=0.008), with significant reductions seen with both doses of rivaroxaban. Critically, rivaroxaban 2.5 mg bid (but not 5 mg bid) reduced the risk of cardiovascular and all-cause mortality by 34% and 32%, respectively, compared with placebo. Primary efficacy endpoint findings with rivaroxaban were consistent among all ACS types and major patient subgroups analysed. Rivaroxaban increased the rate of noncoronary artery bypass graft-related TIMI major bleeding almost fourfold (2.1% vs. 0.6%; HR 3.96; 95% CI: 2.46-6.38; p<0.001) and intracranial haemorrhage more than threefold (0.6% vs. 0.2%; HR 3.28; 95% CI: 1.28-8.42; p=0.009) compared with placebo. However, neither dose showed a significant increase in fatal bleeding compared with placebo (0.3% [overall] vs. 0.2%; p=0.66). Although an analysis of stent thrombosis was not part of the formal statistical hierarchy, rivaroxaban 2.5 mg bid reduced the risk of stent thrombosis by 35% compared with placebo.
Based on the findings of the ATLAS ACS 2 TIMI 51 study, rivaroxaban 2.5 mg bid was recently approved by the European Commission for the prevention of atherothrombotic events (cardiovascular death, MI or stroke) after an ACS event in adult patients with elevated cardiac biomarkers in combination with standard antiplatelet therapy38.
IMPLICATIONS FOR ANTICOAGULANT DOSING
What factors account for the differences in the outcomes of these two phase III trials, APPRAISE-2 and ATLAS ACS 2 TIMI 51? One possible explanation is the difference in patient populations: APPRAISE-2 patients were older and had more comorbidities (e.g., diabetes, renal dysfunction) than those in ATLAS ACS 2 TIMI 51. The rivaroxaban trial excluded patients with prior stroke or transient ischaemic attack who received both ASA and a thienopyridine, because results from previous studies suggested that they would not benefit from more intense thromboprophylaxis39,40. Based on available data, these higher-risk patients included in APPRAISE-2 may have responded differently to the intensified antithrombotic therapy41.
Dosing differences provide another likely explanation for the variation in results between APPRAISE-2 and ATLAS ACS 2 TIMI 51. In APPRAISE-2, the total daily doses used were the same as those used for stroke prevention in patients with atrial fibrillation (AF) in the phase III ARISTOTLE42 and AVERROES43 trials (5.0 mg bid). In contrast, in the ATLAS ACS 2 TIMI 51 study, two low doses were selected for evaluation, and the dose that exhibited the best efficacy (2.5 mg bid) was only one quarter of the total daily dose used for stroke prevention in patients with AF (20 mg od)41. The regimen used in ATLAS ACS 2 TIMI 51 was also switched from od, used in the AF trial (ROCKET AF), to bid44.
The importance of dose selection in ACS could be, in part, because of the “dual nature” of thrombin. Thrombin can both promote and inhibit coagulation, depending on its concentration. With low thrombin concentrations, anticoagulant activity prevails, acting through thrombomodulin and activating protein C. At higher thrombin concentrations, the procoagulant activity of thrombin prevails through platelet activation, fibrinogen transformation to fibrin and activation of Factors V and VIII45. This dual nature of thrombin is often described as the “thrombin paradox” (Figure 2).
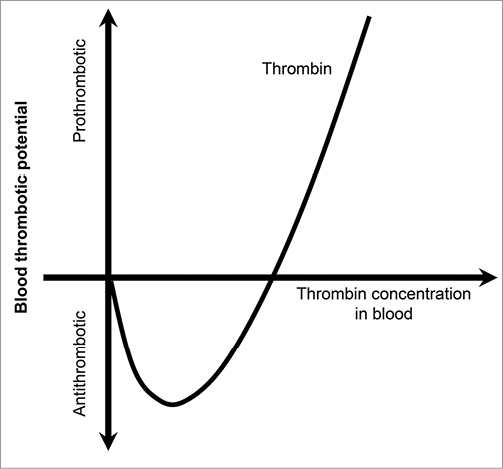
Figure 2. The “thrombin paradox”. Thrombin can both promote and prevent blood clotting. Scales for both axes are given in arbitrary units. Adapted with permission from Macmillan Publishers Ltd: Nature (Griffin JH, vol. 378 pages 337-338), copyright (1995)45.
Correlation between the level of thrombin generation and clinical events in patients with ACS from the GUSTO IIb trial, as reported by Ardissino and colleagues46, supports this hypothesis. In line with an earlier study11, a heightened degree of thrombin generation (as assessed by prothrombin fragment 1+2 production) persisted in the 12 months after an ACS event. However, this subsequent study showed that the relative level of thrombin generation over time was not linearly correlated with the clinical events; instead, a U-shaped curve existed, in which higher rates of the primary efficacy endpoint (cardiac death or MI) were seen at both very low and very high fragment 1+ 2 levels. This suggests that, for a given anticoagulant to be effective for secondary prevention of ACS, the “optimal” dose must be found.
In ATLAS ACS 2 TIMI 51, the lower rivaroxaban dose evaluated achieved more favourable results, significantly reducing mortality. This may simply be a result of the deleterious effects of bleeding, particularly because the primary endpoint of this trial included bleeding-related death; greater bleeding with higher doses may result in more events, as may have been the case in APPRAISE-2. However, lower anticoagulant doses have previously performed better than higher doses, in particular in the phase II PENTUA trial47, in which four pentasaccharide doses were tested against conventional therapy in patients with NSTEMI-ACS. The lowest dose was shown to be more efficacious than the other three doses and resulted in less bleeding.
Taken together, these observations not only support the concept that the thrombin paradox may play a role in the physiological regulation of haemostatic balance after an index ACS event, but also support the mechanistic rationale for the success of low-dose rivaroxaban in the ATLAS ACS 2 TIMI 51 study. Furthermore, the fact that the lower dose of rivaroxaban translated into a significant reduction in cardiovascular and all-cause mortality suggests that a re-evaluation of the thrombin hypothesis may be in order.
Potential impact of ATLAS ACS 2 TIMI 51 trial findings on clinical practice
The positive outcomes of ATLAS ACS 2 TIMI 51 have shown that a balance between efficacy and safety can be achieved in patients with ACS using a low-dose anticoagulant with DAPT. Historically, demonstration of a clinical benefit of long-term oral anticoagulant therapy in the ACS setting has been lacking, and adopting a dual pathway strategy for secondary ACS prevention will require a change in the mindset of cardiologists – concern over excessive bleeding from combining anticoagulants with antiplatelets is firmly entrenched. Nevertheless, findings from ATLAS ACS 2 TIMI 51, particularly with regard to reduced mortality, suggest that rivaroxaban may have a substantial impact on the outcomes of patients with ACS in the clinical setting. Furthermore, this is reflected by the European Commission’s recent decision to approve rivaroxaban for patients with ACS and elevated biomarkers38. Given the importance of the optimal anticoagulant dose, the use of other newer oral anticoagulants may be revisited with trials of lower doses. Further to the more potent antiplatelet agents, prasugrel and ticagrelor, agents such as rivaroxaban could become another important addition to the cardiologist’s armamentarium in the fight against recurrent ACS. Permitted antiplatelet therapies in ATLAS ACS 2 TIMI 51 were based on guidelines and approvals at the time the study was initiated, when neither prasugrel nor ticagrelor was approved. As a result, standard-care antiplatelet therapy was limited to ASA with or without clopidogrel (or ticlopidine). Therefore, it is not yet known whether the combination of rivaroxaban and either prasugrel or ticagrelor would provide a positive benefit–risk ratio in patients with ACS. Now that prasugrel and ticagrelor are both approved and used in clinical practice, future studies are required to answer this question.
In real-world clinical practice, some patients will no doubt have both ACS and AF concomitantly. Given that “low-dose” rivaroxaban (2.5 mg bid) was studied in ATLAS ACS 2 TIMI 51 in patients with ACS (but no AF), and high-dose rivaroxaban (20 mg od) in ROCKET AF in patients with AF (but no ACS), an additional question for future clinical practice would be how to treat patients with AF receiving rivaroxaban who have an ACS event. The recently announced PIONEER AF-PCI trial will hopefully help to address this question48. Following stent placement, patients with AF will be treated with either rivaroxaban 2.5 mg bid or VKA (target INR 2.0-3.0) for the duration of DAPT (one, six or 12 months), followed by rivaroxaban 15 mg od (10 mg od in patients with moderate renal impairment) or VKA (target INR 2.0-3.0), respectively, plus ASA for the remainder of the study (up to 12 months). Alternatively, patients in a third treatment arm will receive rivaroxaban 15 mg od plus clopidogrel (without ASA) for the entire 12-month study period. Prasugrel and ticagrelor are also permitted to be used in PIONEER AF-PCI in a certain number of patients as alternatives to clopidogrel; therefore, this study will also provide valuable information regarding their use with rivaroxaban. Following completion of ATLAS ACS 2 TIMI 51, further studies of rivaroxaban in patients with coronary and peripheral artery disease (COMPASS)49 and patients with heart failure (COMMANDER HF)50 have also been announced. As these studies, and PIONEER AF-PCI, are completed in the upcoming years, the full implications of the ATLAS ACS 2 TIMI 51 study on future clinical practice in cardiology will unfold.
Acknowledgements
The author would like to acknowledge Geraint Owens and Joanne McGrail who provided medical writing services with funding from Bayer HealthCare Pharmaceuticals and Janssen Research & Development, LLC.
Conflict of interest statement
Advisory board membership for AstraZeneca and service on speakers’ bureaux for AstraZeneca, Bayer, GlaxoSmithKline and Eli Lilly.

