
Abstract
Aims: Functional assessment of non-culprit lesions (NCL) in patients presenting with ST-elevation myocardial infarction (STEMI) and multivessel disease constitutes an unmet need. This study aimed to evaluate the diagnostic accuracy of quantitative flow ratio (QFR) in the functional assessment of NCL during the acute phase of STEMI.
Methods and results: This was a retrospective, observational, multicentre study including patients with STEMI and staged fractional flow reserve (FFR) assessment of NCL. QFR in NCL was calculated from the coronary angiogram acquired during primary PCI in a blinded fashion with respect to FFR. The diagnostic value of QFR in the STEMI population was compared with a propensity score-matched population of stable angina patients. Eighty-two patients (91 NCL) were included. Target lesions were of both angiographic and functional (mean FFR 0.82±0.09) intermediate severity. The diagnostic performance of QFR was high (AUC 0.91 [95% CI: 0.85-0.97]) and similar to that observed in the matched control population (AUC 0.91 vs 0.94, p=0.5). The diagnostic accuracy of QFR was very high (>95%) in those vessels (61.5%) with QFR values out of a ROC-defined “grey zone” (0.75-0.85). A hybrid FFR/QFR approach (FFR only when QFR is in the grey zone) would adequately classify 96.7% of NCL, avoiding 58.5% of repeat diagnostic procedures.
Conclusions: QFR has a good diagnostic accuracy in assessing the functional relevance of NCL during primary PCI, similar to the accuracy observed in stable patients.
Introduction
Around 40-50% of patients presenting with acute ST-elevation myocardial infarction (STEMI) present with significant multivessel coronary artery disease (MVD)1. Recently, two large trials2,3 have shown a clinical benefit of a complete fractional flow reserve (FFR)-guided revascularisation of non-culprit lesions (NCL) compared with a strategy of revascularisation restricted to the infarct-related artery. However, even when considering NCL interrogation at a staged procedure in the subacute STEMI phase, repeated invasive procedures and the associated risks, the cost of pressure wires, hyperaemic agents, inadequate financial reimbursement and time constraints within the catheterisation laboratory are potential obstacles.
All of the above justify the need to obtain functional information on NCL during primary PCI, without additional intracoronary instrumentation, using a standardised, reproducible method. Functional coronary imaging indices based on computational fluid dynamics (CFD) are potential candidates for this role. These indices have the potential to discriminate functionally significant lesions non-invasively while avoiding intracoronary instrumentation and administration of adenosine, thus not interfering with the workflow of primary PCI in STEMI. One of these novel indices is quantitative flow ratio (QFR), an adenosine-free angiography-based method that allows fast online computation of FFR4. Evidence of good agreement between QFR and FFR is becoming increasingly available for patients with stable coronary artery disease (CAD)4,5,6,7,8,9,10; however, data regarding its potential role in the functional assessment of NCL in patients with STEMI remain limited11,12.
The present study aimed to investigate the reliability of QFR for assessing NCL at the time of STEMI presentation and primary PCI, comparing it against FFR measured in a staged procedure as the reference standard.
Methods
STUDY DESIGN
This was a retrospective, observational, multicentre, international study. Patients were enrolled at three tertiary centres in three different countries: Hospital Clínico Universitario San Carlos (Madrid, Spain), King’s College Hospital (London, United Kingdom) and Toda Chuo General Hospital (Toda, Japan). QFR in NCL was performed at a blinded core laboratory using the coronary angiogram obtained during primary PCI, and its diagnostic performance was compared with that of a matched control group of stable angina patients, using FFR as the reference standard (Figure 1).
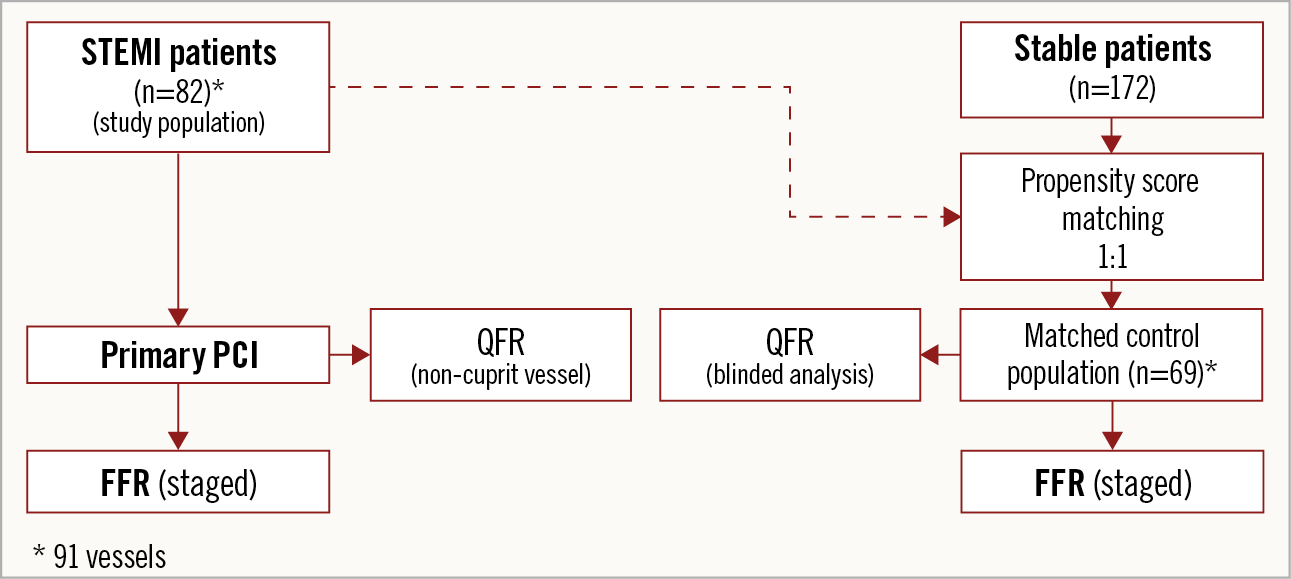
Figure 1. Study flow chart. FFR: fractional flow reserve; PCI: percutaneous coronary intervention; QFR: quantitative flow ratio; STEMI: ST-elevation myocardial infarction
STUDY POPULATION
Patients ≥18 years of age with confirmed STEMI receiving primary PCI within a maximum of 12 hours after symptom onset were assessed for eligibility. All NCL in non-infarct-related vessels (≥2.0 mm) that were subsequently assessed with FFR in a second procedure were included in the analysis.
The exclusion criteria were left main (LM) and ostial right coronary artery (RCA) target lesions, previous bypass grafting involving a non-infarcted territory and absence of coronary angiography calibration metadata. Moreover, bifurcation lesions were excluded because of specific limitations of the present QFR application. Finally, patients fulfilling inclusion criteria were excluded in case of inadequate image quality of the angiography, vessel overlapping or tortuosity.
DATA COLLECTION
Patient baseline demographics, clinical and procedural characteristics were collected and input into a dedicated electronic database by the co-investigators of the participating centres. Coronary angiograms obtained at the time of primary angioplasty were sent to the core laboratory at Hospital Clinico Universitario San Carlos, where they were anonymised for QFR analysis. Those performing analysis were blinded to FFR values and final management.
FRACTIONAL FLOW RESERVE
As per study criteria, FFR-guided revascularisation of NCL was performed in a staged procedure after primary PCI, either during initial hospitalisation or at scheduled follow-up. FFR was measured according to a state-of-the-art practice in all three participating centres, using intravenous adenosine as the standard. FFR was defined as the lower ratio between the mean distal coronary pressure and the mean aortic pressure during steady-state maximum hyperaemia and was considered potentially flow-limiting if ≤0.80.
QUANTITATIVE FLOW RATIO
QFR is an angiography-based method of calculating FFR using anatomical and functional principles. Therefore, its values are equivalent to the FFR scale of grading severity (i.e., from 0 to 1, with a cut-off point of ≤0.80)13. QFR is calculated by applying CFD to three-dimensional quantitative coronary angiography (3D-QCA) derived from two angiographic projections. Additionally, QFR incorporates a flow-dependent individualised adjustment based on Thrombolysis In Myocardial Infarction (TIMI) frame count9. In this study, 3D-QCA and QFR were obtained using QAngio XA 3D software (Medis, Leiden, the Netherlands). Briefly, QFR analysis was performed as follows: two angiographic projections separated by at least 25 degrees were selected from coronary angiography performed at the time of primary PCI to obtain a 3D reconstruction of each target coronary artery (≤1 minute). The distal boundary of the analysed vessel segment was marked according to the original position of the pressure-wire sensor during the staged FFR assessment (≃1 minute). After manual corrections of any gross deviation of the automatic vessel reconstruction from the true vessel contour (≃3-5 minutes), 3D-QCA was automatically obtained. 3D-QCA-derived diameter stenosis (DS) was the parameter of choice to describe angiographic stenosis severity. Finally, the flow correction based on TIMI frame count was added to obtain the final QFR value (≤1 minute) (Figure 2). As a reference, in the FAVOR II Europe-Japan Study, time to complete QFR analysis was five minutes (interquartile range, 3.5–6.1), significantly shorter than the time to perform FFR10. However, QFR analysis was not achievable when the available projections and image quality of the angiography did not allow visual estimation of target segments in at least two valid projections (i.e., overlapping, tortuosity) and/or TIMI frame count analysis (inadequate contrast opacification). Moreover, absence of a healthy proximal reference diameter (i.e., LM and RCA ostial lesion) limited QFR analysis because of lack of data in this scenario.
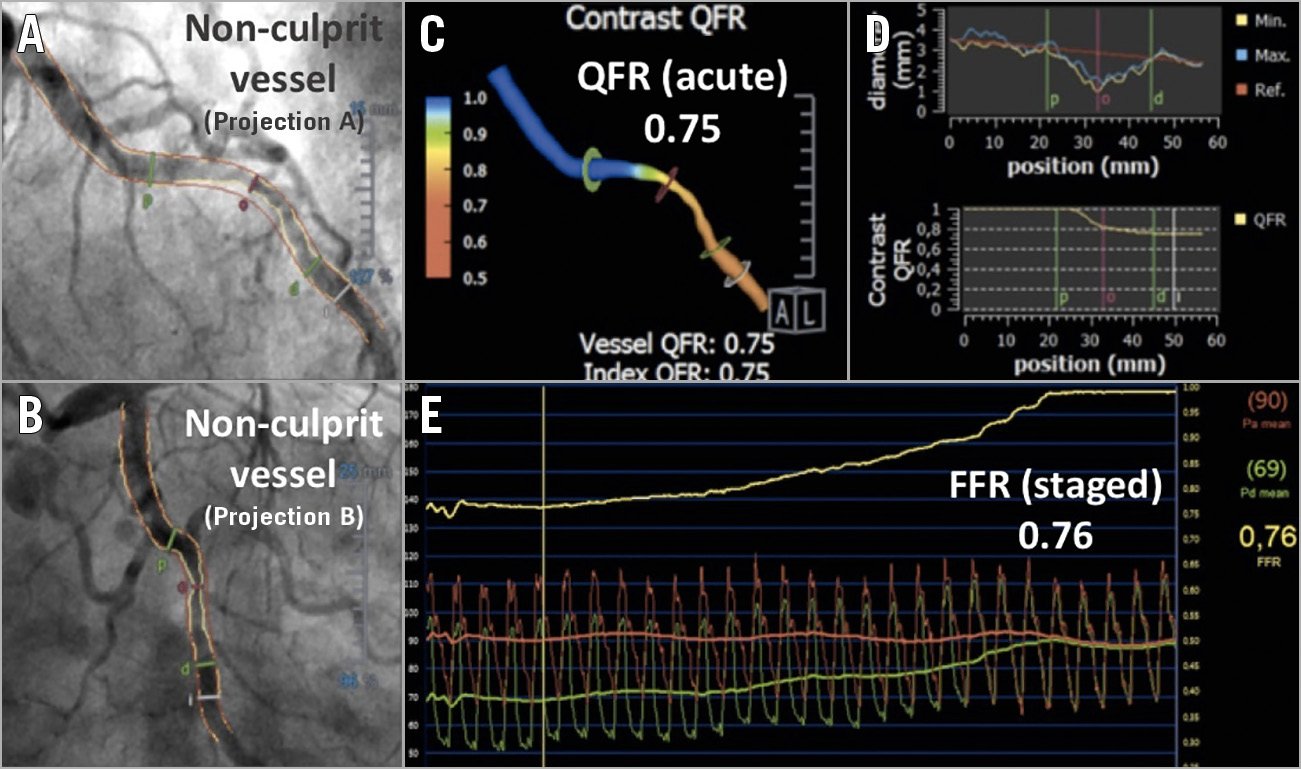
Figure 2. Non-culprit vessel acute QFR and staged FFR functional assessment. A) & B) A non-culprit lesion in circumflex artery assessed by QFR in the acute phase of STEMI. C) A three-dimensional reconstruction of the vessel with colour mapping of the pressure drop. D) Vessel diameter graph and QFR decrease along the vessel length. E) FFR pullback of the same vessel, obtained five days later. FFR: fractional flow reserve; QFR: quantitative flow ratio; STEMI: ST-elevation myocardial infarction
QFR analysis was performed by a recognised analyst who passed the certification process strongly recommended for any aspiring QFR analyst and managed by Medis.
Finally, free licence of QAngio XA 3D software was provided by the company.
STATISTICAL ANALYSIS
Continuous variables are expressed as mean±standard deviation (SD) or median with interquartile range (IQR), as appropriate. Normality was tested with the Shapiro-Wilk test, P-P and box plots. Categorical variables are presented as numbers and percentages. Diagnostic performance of QFR was assessed with the area under the receiver operating characteristic (ROC) curve (AUC), taking FFR as the reference. The relationship and agreement between QFR and FFR were assessed by Pearson correlation coefficient and Bland-Altman plot, respectively. The ROC analysis was used to outline a grey zone in which a drop in diagnostic accuracy of QFR was noted, identifying upper and lower QFR boundaries with a diagnostic accuracy >95%. All analyses were performed with Stata 13 software (StataCorp LP, College Station, TX, USA). A p-value <0.05 was considered statistically significant.
In order to have a reference of QFR diagnostic performance, a cohort of 172 patients with stable angina (206 target vessels) who underwent FFR assessment was used as a comparator. The control group was selected by propensity score matching, according to the same selection criteria of the study population, using a 1:1 nearest neighbour pairing based on a logistic regression model including the following covariates: interrogated artery, diameter of the stenosis and reference vessel diameter. Propensity score matching was performed using the MatchIt package of R software (R Foundation for Statistical Computing, Vienna, Austria). The diagnostic performance of QFR in the STEMI group was compared with that of the resultant matched control population as per comparison of AUC performed with the DeLong test.
Results
BASELINE CLINICAL AND LESION CHARACTERISTICS
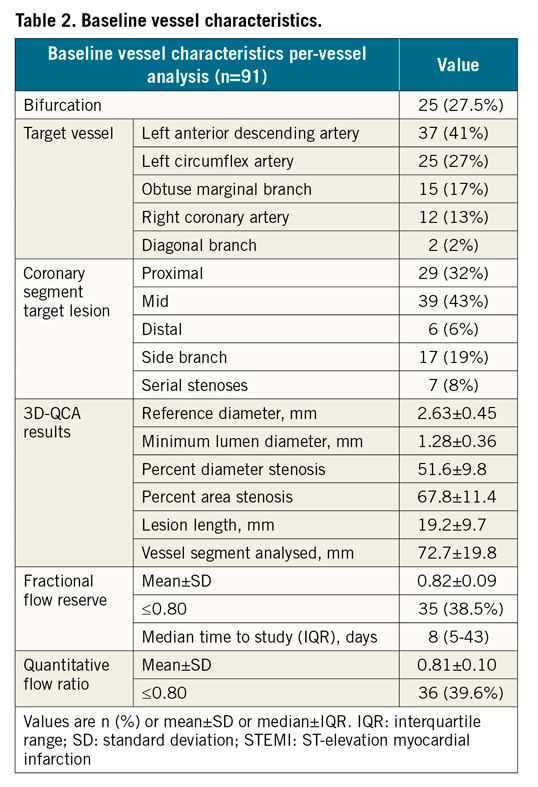
A total of 136 patients (159 vessels) fulfilled the inclusion criteria and, after screening for inadequate image quality of the angiography (1 vessel), excessive vessel overlapping or tortuosity (36 vessels), diffuse disease (3) and absence of valid projections (28 vessels), 82 patients (91 vessels) were finally included. Table 1 summarises the baseline clinical characteristics of the STEMI population enrolled in the study. In all patients, the culprit lesion was treated with stent implantation.
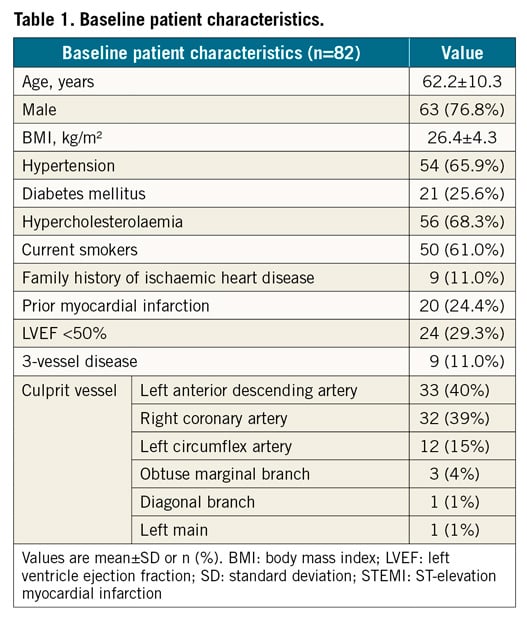
The left anterior descending was the most commonly interrogated vessel (37 [41%]). On average, stenoses had intermediate angiographic severity (51.63±9.76 %DS) (Table 2).
FRACTIONAL FLOW RESERVE
FFR values were normally distributed around a mean value of 0.82±0.09 (Table 2, Supplementary Figure 1). Minimal and maximal FFR values were 0.56 and 0.97. In a majority of cases (51%), FFR measurements were obtained within the first 10 days, with a median of 8 days after the initial PCI procedure (IQR 5–43). All cases with FFR ≤0.80 were treated with PCI.
OVERALL ACCURACY OF QUANTITATIVE FLOW RATIO
Like FFR, QFR presented a normal distribution with a mean of 0.81±0.09 (min-max 0.51-0.97), denoting intermediate stenosis severity (Table 2, Supplementary Figure 1). Diagnostic performance of QFR in detecting ischaemia-generating lesions (FFR ≤0.80) was high as per ROC analysis (AUC 0.91 [95% CI: 0.85-0.97]), and significantly better than DS (AUC 0.73 [95% CI: 0.62-0.84], p<0.001) (Supplementary Figure 2). The correlation (r=0.75 [p<0.001]) and agreement (mean difference −0.004 [95% CI: −0.032 to 0.023]) between QFR and FFR were strong (Figure 3). Sensitivity and specificity of QFR to determine the FFR-based functional stenosis severity were 85.7% and 80.0%, respectively, with a Youden index of 0.66 (Supplementary Table 1). Negative and positive predictive values were 87.3% and 77.8%, respectively. Classification agreement (i.e., diagnostic accuracy) between QFR and FFR was observed in 83.5% of the vessels.
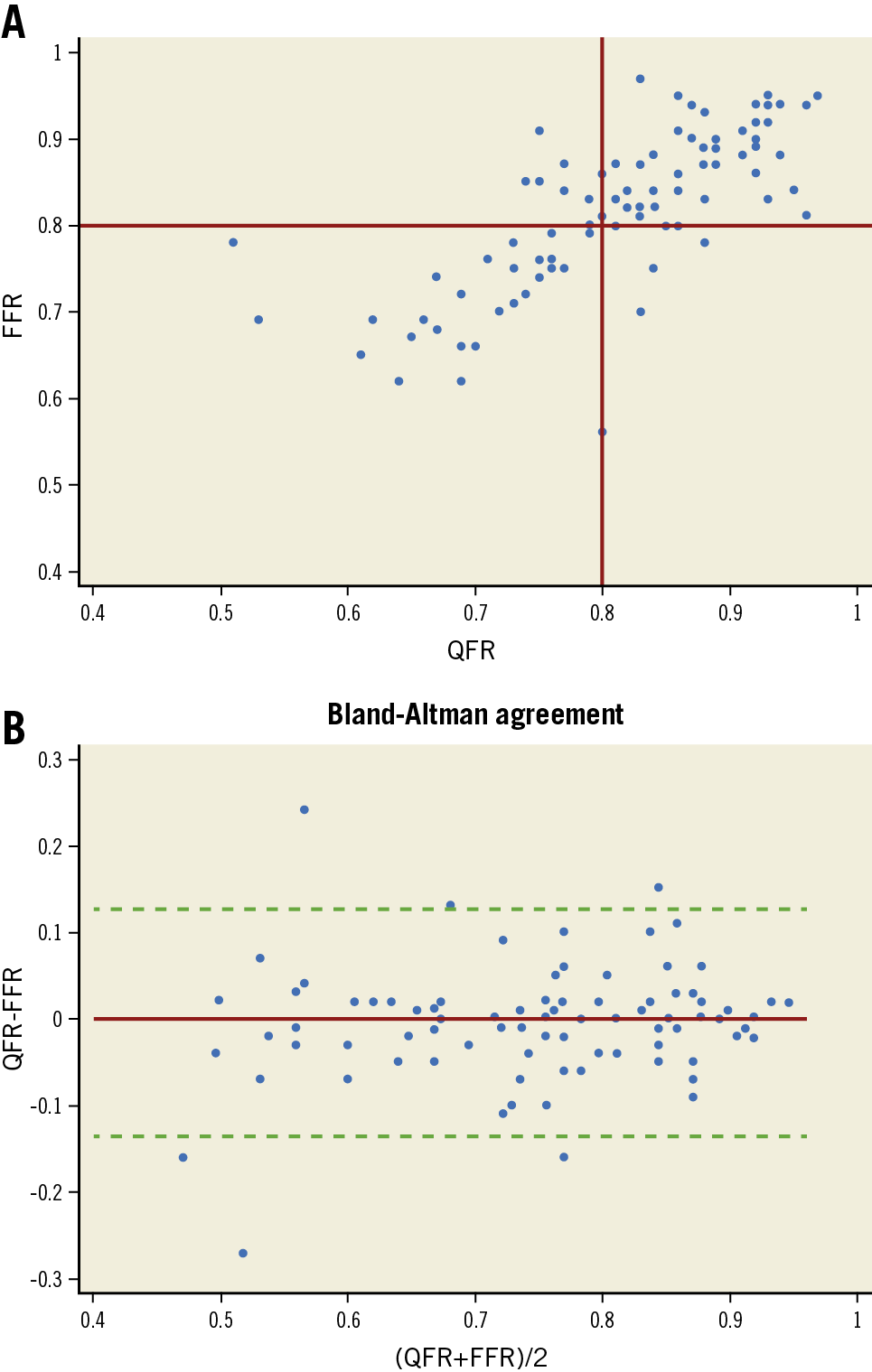
Figure 3. Relationship and agreement between QFR and FFR. A) Scatter plot showing the correspondence of QFR and FFR. Regression graph with confidence interval shows the correlation. B) Bland-Altman plot of differences between QFR and FFR. The majority of the values are included within +/- 2SD of the mean difference. FFR: fractional flow reserve; QFR: quantitative flow ratio; SD: standard deviation
QFR GREY ZONE
The agreement in functional stenosis classification between QFR and FFR was above 95% for QFR values <0.75 and >0.85. In that range of QFR values (56 vessels [61.5%]), the performance and accuracy of QFR were very high (AUC 0.98 [95% CI: 0.95-1.00], classification agreement 95% [53/56] [p<0.001 for comparison of accuracy]). On the other hand, inside the QFR grey zone defined for values ≥0.75 and ≤0.85 (35 vessels [38.5%]), performance and accuracy of QFR were modest (AUC 0.63 [95% CI: 0.42-0.84], classification agreement 68.6%) (Supplementary Figure 3).
The use of a hybrid strategy combining decision making based solely on QFR when the values are out of the grey zone, and FFR only in cases with QFR values within the grey zone, would have avoided the use of intracoronary physiology in 56 vessels (61.5%). In our study population, this hybrid QFR/FFR strategy could have avoided repeat catheterisation and FFR measurements in 48 patients (58.5%).
COMPARISON WITH STABLE CAD
The propensity score model yielded 91 vessels from 69 patients with stable CAD (Table 3). Overall, the patient and vessel characteristics of the reference group were similar to the STEMI study population, although significantly more patients with diabetes mellitus and fewer active smokers were noted in the control group. None of these clinical variables has been noted to influence QFR-FFR agreement in previous studies12. Similarity in the angiographic characteristics reflected a good adjustment of the propensity score model. Mean FFR and QFR values were also similar. The numerical diagnostic value of QFR compared to FFR as the reference standard improved slightly in the stable CAD group, although the differences were not statistically significant (Table 3, Figure 4).
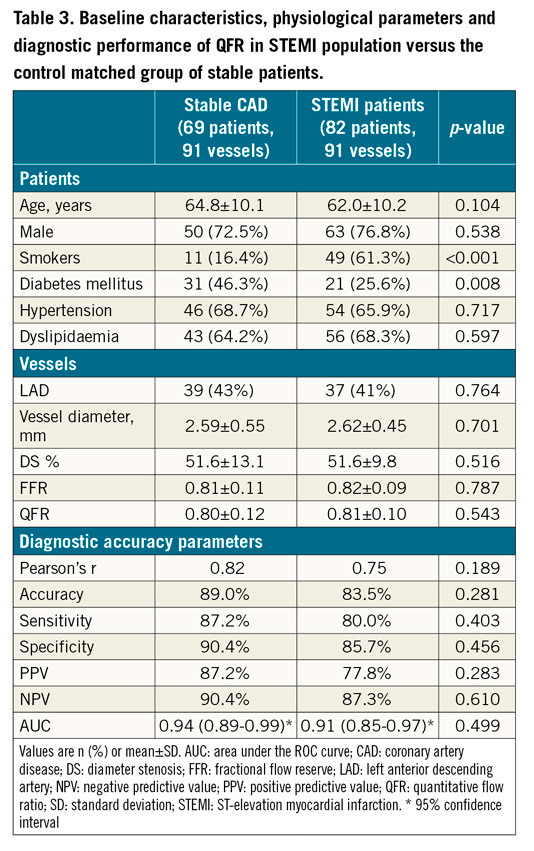
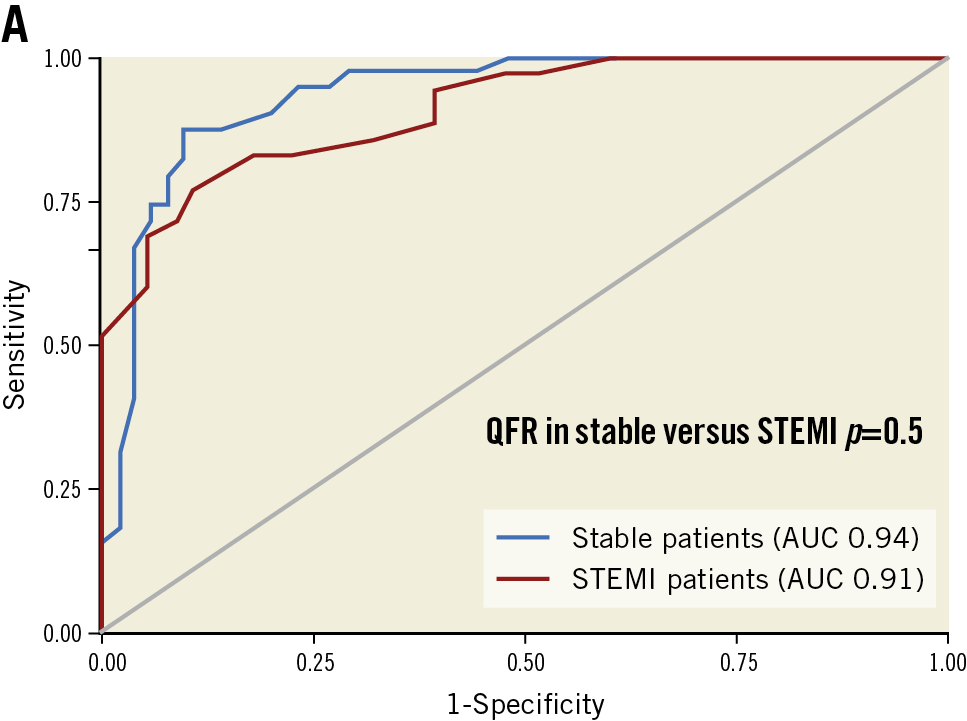
Figure 4. Comparison of QFR diagnostic performance in stable CAD and STEMI populations. QFR applied to non-culprit vessels at the time of primary PCI showed comparable diagnostic performance compared to QFR applied to stable coronary disease. AUC: area under the curve; QFR: quantitative flow ratio; STEMI: ST-elevation myocardial infarction
Discussion
In this study, we found that functional assessment of NCL using QFR during primary PCI is feasible, with a classification concordance with FFR measurements similar to that observed in stable patients. When QFR is used as part of a hybrid QFR-FFR strategy, adequate functional classification can be achieved at the time of primary PCI in 96.7% of NCL and could have avoided further invasive testing in 58.5% of patients.
Recent studies on the management of MVD in patients presenting with STEMI have highlighted the potential value of FFR-guided revascularisation applied to NCL. As in stable patients, physiology may serve as a valuable gatekeeper for unneeded PCI. In this regard, the Compare-Acute trial2 showed that FFR assessment of NCL in the acute phase of STEMI shifted more than 50% of angiography-based indications from PCI to PCI deferral. The potential of QFR as an alternative to FFR interrogation of NCL stems from the fact that it can be easily performed during or after primary PCI, does not require coronary instrumentation, and does not need administration of potent coronary vasodilators. The implications are that QFR might contribute to streamlining the care of patients with STEMI and MVD, sparing additional invasive procedures, and reducing risks, costs and length of hospital stay.
In our study, the diagnostic accuracy parameters of QFR when used in the acute setting of STEMI for discriminating functionally significant NCL were high, although generally lower than those reported in previous studies including patients with stable CAD (Supplementary Table 2),4,5,6,7,8,9,10. This could be due to the intrinsic limitations of obtaining angiograms during an acute presentation that could potentially affect QFR accuracy in this clinical setting. Our data confirm the results obtained by Sejr-Hansen et al12 who describe the same overall classification agreement with FFR (84%).
USE OF A HYBRID APPROACH OF QFR AND FFR IN CLINICAL DECISION MAKING
To date, the non-inferiority of QFR, compared to FFR, in terms of safety of clinical decision making, has not been demonstrated. An alternative method of gathering evidence on the clinical utility of QFR is using a hybrid QFR-FFR approach, in which clinical decisions based on QFR are limited to values below and above a central “FFR zone”.
In our study, we found that applying a hybrid approach with QFR values <0.75 or >0.85, limiting FFR interrogation to NCL with QFR values within these boundaries, would result in an overall classification agreement of 96.7% (Figure 5). These values are superior to those documented in the ADVISE II trial13 applying an iFR/FFR hybrid approach which demonstrated classification agreement of 94.2%. Of note, assuming that scheduled FFR in the subacute phase would be the routine practice to assess the functional relevance of NCL in patients with STEMI and MVD, the use of a hybrid QFR/FFR approach, based on staged FFR interrogation only in non-culprit vessels with QFR within the grey zone (0.75-0.85), could be valuable in terms of reduction of healthcare costs. This is mainly due to avoidance of pressure wires and adenosine in patients with only very positive and/or very negative QFR values (58.5% of the sample size) and even no need of a second procedure in patients with only very negative QFR values (35% of the sample size).
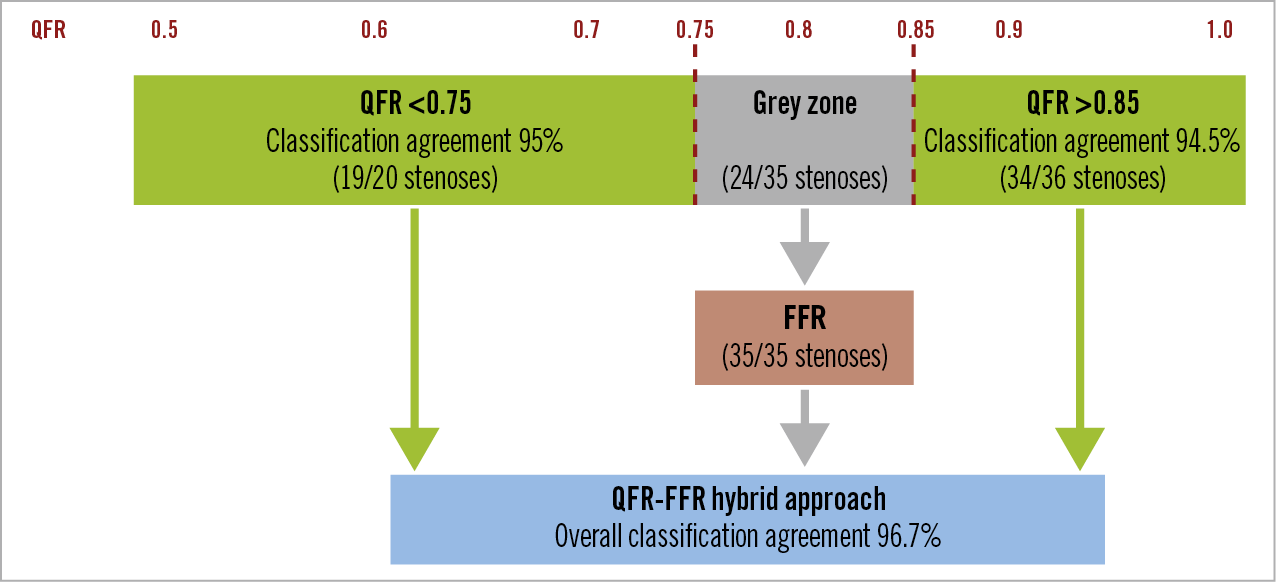
Figure 5. QFR-FFR hybrid approach strategy. The QFR treatment (<0.75) and deferral (>0.85) values correctly classified 95% and 94.5% of stenoses, respectively. After including standard classification with FFR (virtual classification agreement 100%) in-between, the overall classification agreement of the proposed QFR-FFR hybrid approach increased to 96.7%. FFR: fractional flow reserve; QFR: quantitative flow ratio
Unfortunately, cost-effectiveness analysis was not possible, as the QAngio software licence fee is still not definite due to the fact that the software is still in its launch phase.
Study limitations
Our study had several limitations. As a consequence of the retrospective design of this study, some vessels had to be excluded because of insufficient angiographic image quality, not suitable for QFR computation, and, in most of the cases, absence of calibration data of coronary angiography due to incompatibility of QAngio XA 3D software with old X-ray software. Moreover, although nitrates are regularly administered before coronary angiography, this could not happen in the acute phase of STEMI due to potential haemodynamic instability, leading to potential underestimation of coronary diameters and consequently to lower values of QFR. However, the positive predictive value of QFR in our study was high and comparable to previous studies and therefore reassuring about the minor impact of this limitation. Moreover, a higher prevalence of microvascular dysfunction in non-culprit vessels of patients with STEMI is still a matter of debate9,14,15. Consequently, a reduced QFR accuracy due to microvascular dysfunction, as described elsewhere9, cannot be excluded. Prospective studies with angiographic projections dedicated to QFR analysis are likely to overcome these limitations (in FAVOR II Europe-Japan10, prospective QFR assessment was achievable in 296 of 302 vessels with the exclusion of six vessels only) and reproduce our findings. Finally, future trials randomising strategies (FFR vs hybrid QFR-FFR) with clinical follow-up should test the non-inferiority of this novel approach.
Conclusions
QFR has a high diagnostic accuracy in assessing the functional stenosis relevance of NCL, as judged by FFR, when applied to angiography acquired during primary PCI in patients with STEMI. A QFR-FFR hybrid approach for NCL, with need of invasive functional assessment only for QFR values ≥0.75 and ≤0.85, could be safe and cost-effective. Prospective randomised trials including clinical follow-up data are needed to confirm QFR as a proper diagnostic tool and as a predictor of clinical events in this clinical situation.
|
Impact on daily practice Functional assessment of non-culprit lesions (NCL) in patients presenting with STEMI and multivessel disease is underperformed due to practical considerations such as financial reimbursement and the additional burden to the operator. QFR, in particular within a QFR-FFR hybrid approach, has been shown to have an acceptable diagnostic performance in fast assessment of NCL without additional procedures and costs (besides the price of the annual QFR software licence), in a considerable number of patients. |
Appendix. Study collaborators
Nieves Gonzalo, MD, PhD, Hospital Clínico San Carlos, Madrid, Spain; Pablo Salinas, MD, PhD, Hospital Clínico San Carlos, Madrid, Spain; Luis Nombela-Franco, MD, PhD, Hospital Clínico San Carlos, Madrid, Spain; Ivan Nuñez-Gil, MD, PhD, Hospital Clínico San Carlos, Madrid, Spain; Maria Del Trigo, MD, PhD, Hospital Clínico San Carlos, Madrid, Spain; Pilar Jimenez-Quevedo, MD, PhD, Hospital Clinico San Carlos, Madrid, Spain; Jonathan Byrne, MD, King’s College Hospital, London, United Kingdom.
Funding
This project had no dedicated funding. F. Macaya received a grant from Fundación Interhospitalaria Investigación Cardiovascular.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

