Abstract
Patients being considered for ICD therapy are a heterogeneous group.
For the vast majority, who have significant left ventricular impairment, it has become common practice to assess their coronary artery anatomy as a surrogate for ischaemia and/or viability. Such patients are therefore frequently under the care of both electrophysiologists and interventionists. The coronary anatomy often raises the dilemma about whether such patients should undergo revascularisation. If the patients present with angina or in the context of an acute myocardial infarct then this decision is clear cut. By contrast, however, a significant proportion of them have no history to suggest ongoing ischaemia or of recent MI. In conventional practice, therefore, there would be no decisive mandate to offer them revascularisation, especially PCI, in the absence of further objective evidence of ischaemia or viability. A review of the literature in our paper does not resolve this dilemma.
Further observational data are required to help guide cardiologists as to which of these patients will benefit from revascularisation, since in many cases the coronary anatomy is no surrogate for the presence of ischaemia or viability.
The role of the implantable cardioverter defibrillator (ICD) in primary and secondary prevention of life threatening ventricular arrhythmias is now supported by a wealth of data. With a few exceptions1-4, trials have consistently demonstrated that individuals who have marked themselves out as at risk of sudden cardiac death by either prior episodes5-8 or the presence of certain risk factors9-12 experience a survival benefit when treated with ICDs. Prominent among the populations studied in the major trials, either by inclusion criteria9-11 or demographic fact8,12,13, are patients with coronary artery disease (CAD). The aim of this paper is to examine the evidence base for evaluation and revascularisation of CAD in ICD recipients.
There are three main mechanisms by which the ischaemia resulting from CAD may induce arrhythmias. Firstly, acute myocardial ischaemia alters repolarisation characteristics, increased automacity provides a focus for ventricular fibrillation (VF)14 and wave breaks around the ischaemic zone initiate ventricular tachycardia (VT)15. Secondly, completed myocardial infarcts create transitional areas between scar and normal myocardium with slow conduction fulfilling the prerequisites for re-entry and subsequently VT16, the presence of the electrically inert scar creates a break in the depolarisation wave front allowing VT to degenerate into VF15,17. Thirdly, the dilated poorly functioning ventricle that develops by adverse remodelling as a response to scarring, stunning and hibernation in chronic ischaemia is more able to support re-entry circuits and less able to meet the haemodynamic challenges these present18. A co-existent rise in sympathetic tone19 increases irritability of all myocardium thereby the automatic foci with the potential to trigger VF20.
Registry data suggests residual ischaemia is a strong predictor of arrhythmic events in ICD recipients21, revascularisation has the theoretical potential to reduce acute ischaemia mediated arrhythmogenesis and prevent future infarction and remodelling.
Although revascularisation rates were high in many trials involving CAD patients no inclusion criteria mandating investigation or revascularisation were specified in any of the large study programmes (Table 1).
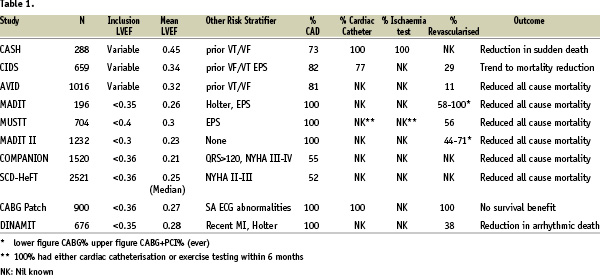
Examining the secondary prevention trials the AVID, CIDS and CASH trials all included a large proportion of CAD patients but only CASH mandated coronary angiography and ischaemia testing. Even in the latter study, it is unclear whether revascularisation was based on functional or anatomical considerations.
Although in primary prevention the MUSTT, MADIT and MADIT II studies included only patients with proven CAD and prevalence of prior revascularisation was high, no requirement for current ischaemia evaluation or revascularisation was stated in the study protocol. Other major primary prevention trials have included patients with ventricular impairment derived from several aetiologies including a large proportion CAD patients, and in common with the previously referenced studies no requirement for ischaemia assessment or revascularisation was included in the study protocols.
Standard methods of ischaemia evaluation are validated against symptomatic or to a lesser extent silent ischaemia but only one study has examined the arrhythmia prone population (a small clinical registry of dobutamine stress echo21). Furthermore most VTs are at supra-physiological rates and as such anatomical lesions which are not haemodynamically significant during stress aimed to mimic physiological situations may become so at higher heart rates, potentially increasing the risk of VT degenerating into VF and reducing efficacy of ICD therapy.
Revascularisation procedures in such patients carry a higher than average risk and it is unlikely that all such patients, regardless of ischaemic burden, will benefit. What investigations to perform and how to act on these results in the primary and secondary prevention of ventricular arrhythmias remain areas open to debate.
The only defibrillator study which mandated revascularisation was the CABG-Patch trial. Inclusion criteria were broadly similar to MADIT II (impaired ventricular function and CAD) with the significant difference that all inclusions were undergoing coronary artery bypass grafting with ICDs implantation at the time of surgery. Results were discordant with other studies in that there was no survival benefit, probably due to an excess of non-arrhythmic deaths in the ICD arm.
In summary, the large randomised trials of ICD cannot be used to answer the question we pose. Limited evidence from outside the major ICD outcome trials provides some insight into the potential benefit of revascularisation among these patients. One report on the role of angiography in patients presenting with VT and temporally remote myocardial infarction demonstrated that arrhythmia recurrence rate was low among those with coronary artery lesions of over 70% of luminal diameter who were subsequently revascularised22. The authors advocate their clinical protocol of diagnostic angiography as primary investigation and revascularisation in such patients with ischemia testing reserved for those found to have equivocal lesions.
Despite the wealth of randomised controlled trials in the field of ICD therapy the optimal strategy for dealing with the commonest predisposing condition for ventricular arrhythmias remains unclear. In primary prevention CABG-Patch might suggest that total revascularisation should be undertaken, and only then, when otherwise indicated after a period of six weeks, the patient reassessed for ICD therapy and treated according to MADIT II criteria. In secondary prevention the picture is even murkier. It is frequently the case that evidence of ischaemia is present in the form of an enzyme rise, chest pain or (post cardioversion) ECG changes rendering it impossible to differentiate cause from effect.
In the absence of a clear cut evidence-based strategy four approaches to ICD candidates not otherwise being revascularised might therefore be considered. First it may be argued that all candidates should be revascularised on anatomical findings at angiography regardless of clinical or objective evidence of ischaemia. Alternatively an ischaemia test may be used to assess both the indication for, and the extent of, revascularisation. Third, others may argue that in the absence of symptoms of ischaemia no further investigation is required and implantation may be carried out without prior evaluation of the ischaemic burden. Finally, in patients with impaired left ventricular function and evidence of ventricular arrhythmias, an ICD might be implanted with evidence of ischaemia sought with a view to revascularisation at a later date.
In the decision making process, which usually involves both electrophysiologist and interventionalist, there are a number of complicating factors. Many individuals have complex coronary disease (often including prior bypass grafting) and in the absence of localising evidence of ischaemia the interventionalist is required to judge culprit from bystander lesions. The threshold for treatment of coronary stenoses in multivessel disease is almost certainly different from other patients treated by the interventional cardiologist and there is a risk that this process has an arbitrary nature, especially where emergency procedures are performed in haemodynamically unstable individuals. In secondary prevention it is often unclear at presentation whether an old infarct has occurred and a post infarct arrhythmia is being detected or an acute ischaemic event precipitated by tachyarrhythmia. Availability, delay and additional cost as well as the lack of appropriate validation of functional ischaemia tests prevent a truly evidence based strategy to investigation and revascularisation pre ICD consideration. The ultimate stage of assessing cost efficacy of such a strategy would require even more extensive data than a clinical endpoint study.
In summary unanswered questions in this field include: Does revascularisation alter arrhythmia burden or prognosis in the ICD population? Do arrhythmias in the context of CAD represent a mandate for revascularisation? What is the post ventricular arrhythmia troponin value? How do we interpret functional ischaemia investigations in arrhythmia patients? What should be the interventional strategy in primary and secondary prevention of ventricular arrhythmias? What are the cost implications the various possible approaches?
The heterogeneity of the patients presenting makes it difficult to envisage a randomised control trial of revascularisation assessment vs. none, other than in the relatively small group of secondary prevention with no impairment of ventricular function and no evidence of ischaemia. Within this group CAD is a less significant player in any case with channelopathies and cardiomyopathies prevalent. In the remaining patients in whom CAD is the major aetiology, systematic observational research is required to document the prevalence and distribution of ongoing ischaemia, to correlate this with the angiographic findings and to document outcomes of revascularisation and number of device therapies post implant.

