Abstract
Background: Virtual histology (VH) uses intravascular ultrasound (IVUS) radiofrequency spectral analysis to locally identify the morphology and composition of atherosclerotic plaques. We sought to explore in vivo the relation between IVUS-derived thin cap fibro-atheroma (IDTCFA) and remodelling index in patients with acute coronary syndromes using IVUS-VH.
Methods and results: Twenty-one patients (63 vessels) were enrolled. When compared to cross sectional areas (CSAs) without necrotic core in contact with the lumen (NCCL), CSAs with NCCL had a larger plaque burden 42.8±11.5% vs. 32.8±11.5%, p<0.001; higher overall necrotic core content [13.8±10.7% vs. 2.3±7.9% (p<0.001)] and calcified tissue [4.7±6.5 vs. 0.66±2.1% (p<0.001)]. On average there were 2 IVUS-derived thin cap fibro-atheroma (IDTCFA) per patient. Nearly half of the IDTCFAs had positive remodelling.
Conclusions: CSAs with NCCL had worse morphological profiles than those with no NCCL. The simultaneous and more detailed assessment of IDTCFA and remodelling index identifies a reduced number of allegedly high-risk plaques. The findings of this study may have important clinical implications, since they shed light into a possible method of identiying potentially high-risk plaques suitable for pharmacological and/or local treatment.
Introduction
For the most part, acute coronary syndromes (ACS) are the consequence of the rupture of particular atherosclerotic plaques called thin-cap fibro-atheromas (TCFAs). These TCFAs have a thin fibrous cap, paucity of smooth muscle cells, heavy inflammatory infiltration of the cap, large necrotic core, positive remodelling and high strain1-6. Coronary plaque rupture is a frequent and unpredictable event that impacts the global burden of cardiovascular disease7. Indeed, coronary heart disease is expected to be the leading cause of disability-adjusted life-years in 20208. Accordingly, many cutting-edge imaging techniques have recently been developed to help us better understand the atherosclerotic process. The first in vivo studies using such techniques were performed against the background of previous histopathological knowledge about morphological and compositional features related to plaques prone to rupture, so several studies have been published recently that mimic previous pathological findings9-11.
Spectral analysis of the IVUS radiofrequency data (IVUS-VH) is emerging as a tool to assess plaque morphology and composition12,13. This combined assessment of remodelling and plaque characterisation might allow a more accurate and complete characterisation in vivo for allegedly high-risk plaques.
We thus sought to further explore in vivo the relation between compositional features of coronary atherosclerotic plaques, specifically IVUS-derived thin cap fibro-atheroma and remodelling index, in patients with acute coronary syndromes.
Methods
Patient selection
From January to May 2005, all patients with acute coronary syndromes admitted for coronary catheterisation and subsequent intervention were eligible if all three coronary vessels were suitable for IVUS interrogation (absence of extensive angiographic calcification and/or severe vessel tortuosity). Acute coronary syndrome encompasses unstable angina (UA) according to the Braunwald classification, non-ST-segment elevation myocardial infarction (NSTEMI) and ST-segment elevation myocardial infarction (STEMI). The three-vessel IVUS-VH acquisition timing was as follows: in patients with UA/NSTEMI, acquisition was performed just after the interventional treatment; in patients suffering from STEMI it was done when the patient was symptom-free, without ECG changes and haemodynamically stable (defined as systolic blood pressure >90 mmHg without vasopressor or inotropic support, and with a heart rate of between 60 and 100 bpm). Informed written consent was obtained from all patients. Our local Ethics Committee approved the protocol.
IVUS-VH acquisition and analysis
Details regarding the validation of the technique on explanted human coronary segments and in vivo post-atherectomy have previously been reported12,13. Briefly, IVUS-VH uses spectral analysis of IVUS radiofrequency data to construct tissue maps that are correlated with a specific spectrum of the radiofrequency signal and assigned colour codes (fibrous [labelled green], fibro-lipidic [labelled greenish-yellow], necrotic core [labelled red] and calcium
[labelled white])12.
The IVUS-VH sampling rate during pull-back is gated to peak R-wave and is therefore dependent on heart rate. For instance, during constant heart rate of 60 bpm, then data will be collected every 0.5 mm.
IVUS B-mode images were reconstructed from the RF data by customised software (IVUSLab Version 4.4, Volcano Therapeutics, Rancho Cordova, CA, USA). Semi-automated contour detection of both lumen and the media-adventitia interface was performed, and the RF data was normalised using a technique known as “Blind Deconvolution”, an iterative algorithm that deconvolves the catheter transfer function from the backscatter, thus accounting for catheter-to-catheter variability14,15. Compositional data was obtained for every slice and expressed as mean percent for each component.
Quantification of the necrotic core in contact with the lumen (NCCL) and its angle was performed using MATLAB® (MathWorks, Natick, MA, USA).
Definitions used in this study
Necrotic core tissue in contact with the lumen, is defined as the presence of necrotic core tissue in direct contact with the luminal space and with no detectable overlying fibrous tissue; this is reported as (i) a continuous variable in mm2, (ii) as a percent of the total plaque composition and (iii) as a percent out of the total necrotic core, (iv) as a binary variable considering the presence or absence of NCCL. In addition, the major confluent pool of NCCL was selectively quantified in terms of area in mm2 and as a percentage of both the total plaque composition and the total necrotic core. The angle (measured from the gravity centre of the lumen) occupied by the entire NCCL was measured as well as the specific angle occupied by the major confluent NCCL. (Figure 1)
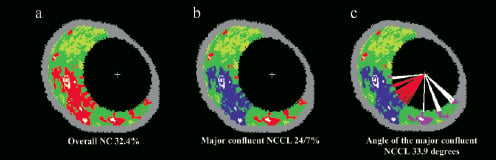
Figure 1. An example of selective quantification of the necrotic core in contact with the lumen. (a) The original IVUS-VH cross-sectional area; (b) Modification using the VH tool to selectively quantify the major confluent necrotic core that is in direct contact with the lumen. (c) Measurement of the angle occupied by the entire necrotic core in contact with the lumen (white and red lines) and specifically the angle occupied by the major confluent necrotic core in contact with the lumen (red lines).
Plaque burden (PB), is defined as EEMarea-Lumenarea/EEMarea X 100, where EEM refers to external elastic membrane.
Remodelling was assessed by means of the remodelling index (RI), expressed as the EEM CSA at the site of minimum luminal area divided by the reference EEM CSA as previously described11,16,17.
The reference site was <10 mm proximal to the selected lesion18. There were no major side branches between the MLA and reference sites. We defined positive remodelling as RI > 1.05 and negative remodelling as RI < 0.95. Values in between were considered neutral (no remodelling).
IVUS-derived TCFA (IDTCFA)9, is defined as a lesion fulfilling the following criteria in at least 3 consecutive CSAs: 1) plaque burden > 40%; 2) confluent necrotic core > 10%19 in direct contact with the lumen in the investigated CSA (Figure 2).
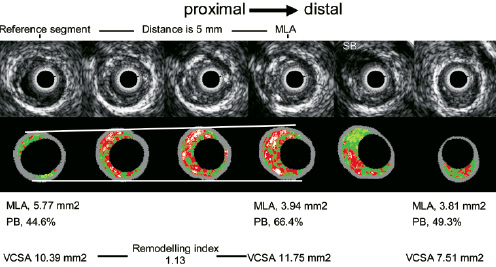
Figure 2. The figure shows the IVUS grey scale and the corresponding IVUS-VH frames of an IVUS derived thin cap fibro-atheroma (four central frames) and the proximal and distal reference segment in which the remodelling index was calculated. MLA, minimum luminal area; PB, plaque burden; VCSA, vessel cross sectional area.
All consecutive CSAs having the same morphologic characteristics were considered as part of the same IDTCFA lesion.
Statistical analysis
Discrete variables are presented as counts and percentages. Continuous variables are presented as means ±SD. A two-sided p value of less than 0.05 indicated statistical significance. Assumptions for normality were checked after transformation based on a p-value >0.20 at Kolmogorov-Smirnov test and by visual assessment of Q-Q plots of residuals. Accordingly, log transformation was performed on the variables with skewed distribution.
To compare CSAs with and without NCCL, in a General Estimating Equations model, with binomial distribution and a logit link function, cases were regarded as a random factor (as we had multiple observations for each patient); we also allowed for an autoregressive correlation structure and the vessel was a fixed factor in the model.
Statistical analyses were performed with use of SPSS software version 11.5.
Results
Twenty-one (63 vessels) consecutive patients were included in this study. The baseline characteristics of the patient population are depicted in Table 1.
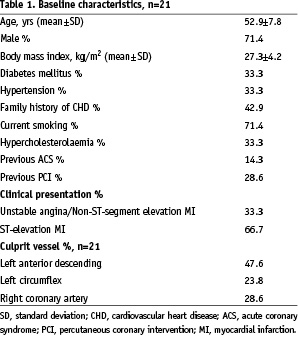
The mean age was 52.9±7.8 years, most being male patients (71.4%). Sixty-six percent of the patients presented with acute myocardial infarction. The culprit vessel was the left anterior descending (LAD) in 47.6%, the left circumflex (LCX) in 23.8% and the right coronary artery (RCA) in 28.6% of the patients. The mean length of the IVUS pull-back was 45.7±22.9 mm.
IVUS-VH findings
A total of 6,351 CSAs were analysed with IVUS-VH. Necrotic core in contact with the lumen was found in 71.7% (4556/6351) of CSAs. These CSAs had a mean area of necrotic core in contact with the lumen of 0.59±0.72 mm2 which corresponds to 13.8±10.7% of the total plaque area; on average 53.8±35.2% of the total amount of necrotic core present in a CSA was in contact with the lumen and the angle occupied by the necrotic core in the luminal circumference was 30.3±34.8°. Nearly all the geometrical and compositional parameters of the CSA with NCCL were significantly different compared with CSAs without necrotic core in contact with the lumen (Table 2).
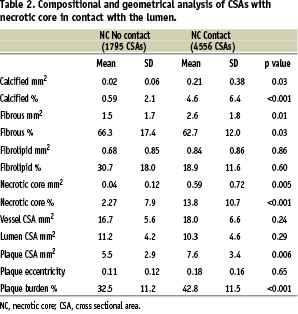
Specifically, CSAs with NCCL had a higher plaque burden compared to CSAs without NCCL, 42.8±11.5% vs. 32.8±11.5%, p<0.001 and more necrotic core and calcified tissue compared to the ones without NC in contact with the lumen, 13.8±10.7% vs. 2.3±7.9% (p<0.001) and 4.6±6.4 vs. 0.59±2.1% (p<0.001) respectively.
Overall, there were 805 (12.7%) CSAs with confluent NCCL >10%; among these only 430 CSAs had a PB >40% (Table 3).
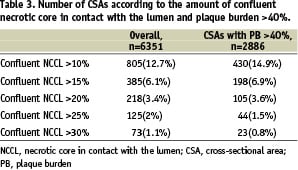
IVUS-derived thin cap fibro-atheroma
A total of 42 IDTCFAs were detected in the 21 patients. However, 13 patients had at least one IDTCFA in their coronary tree (38.1% of the population did not have any IDTCFA). Thus, on average there were 2 IDTCFAs per patient. Seventeen IDTCFAs were found in the LAD, 12 in the LCX and 13 in the RCA. In 7 patients at least one other IDTCFA was found in a different vessel and in 13 vessels more than one IDTCFA was found in the same vessel (Table 4).
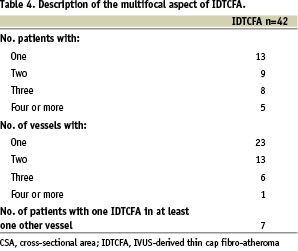
The mean IDTCFA lesion length was 4.8±2.8 mm (Range: 0.95-13.5 mm) and the remodelling index was 1.09±0.21. In three IDTCFAs it was not possible to measure the remodelling index due to lack of reference segment; two of them were located in the proximal LAD, so the only segment without disease proximal to the lesion was the left main artery. Five (11.9%) IDTCFAs had negative remodelling, 14 (33.3%) no remodelling and 20 (47.6%) had positive remodelling. The IDTCFAs were divided into three different categories according to the largest confluent necrotic core in contact with the lumen, largest confluent NCCL < 20%, NCCL between 20-30% and NCCL >30%. Subsequently, the remodelling index was plotted against these three categories (Figure 3).
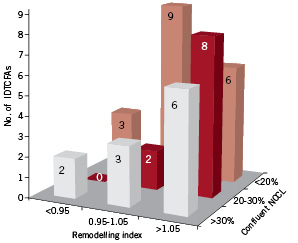
Figure 3. Bar graph shows the number of IVUS-derived thin cap fibro-atheroma according to the largest confluent necrotic core in contact with the lumen (NCCL) and the remodelling index. It seems that among the IDTCFAs there are different degrees of disease, possibly with different likelihoods of rupture.
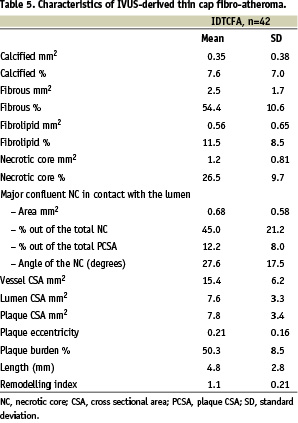
The overall mean necrotic core was 26.5±9.7%, and the major area of confluent NC that was in contact with the lumen was 12.2±8.0% which represented 44.9±21.2% of the total amount of NC present in the CSA. The circumference occupied by the confluent NCCL was 27.6±17.5°. The mean plaque burden was 50.3±8.5, whilst the vessel CSA and lumen CSA were15.4±6.2 mm2 and 7.6±3.3 mm2 respectively (Table 5).
Discussion
The main findings of this study that included 21 patients (63 vessels) with acute coronary syndrome are: 1) on average there were 2 IDTCFAs per patient; 2) after treatment of the culprit lesion, 38.1% of the population did not have any IDTCFA; 3) CSAs with NC in contact with the lumen were more obstructive and with larger vessel CSA than their counterparts without NC in contact with the lumen; 4) CSAs with NC in contact with the lumen had also larger necrotic core and calcified tissue compared to the ones without NC in contact with the lumen; 5) Nearly half of the IDTCFAs had positive remodelling.
To date, there is no single isolated marker of vulnerability that can accurately and precisely identify atherosclerotic plaques at risk of rupture. On the contrary, it seems that the in vivo simultaneous assessment of acknowledged high-risk atherosclerotic plaque characteristics may improve the accuracy to reliably identify vulnerable plaques. For the first time IDTCFA and remodelling index are assessed in an in vivo three vessel IVUS-VH based study in patients with acute coronary syndrome.
Trying to understand the atherosclerosis process has continued for more than a century, and while many paradigms have lost importance, others still remain. Specifically, although vast information with respect to the pathophysiology of acute coronary syndromes has been published, only a few concepts are today generally accepted. First, the former belief that acute coronary syndromes originate exclusively from flow-limiting stenoses has shifted to well-defined histological plaque phenotypes. These plaques have particular characteristics, such as large necrotic core, thin fibrous cap, inflammation within the cap and positive remodelling4,12,20-22. Nevertheless, to date, the natural history of lesions with these characteristics remains unknown and the limited knowledge about their eventual prognosis is provided by retrospective histopathological studies7,23. Second, inflammation plays an important part in the development and growth of such plaques, and most importantly in triggering unpredictable rupturing events24. Third, the multifocal distribution of vulnerable plaques has been proven by means of pathology1, angiography25, angioscopy20, IVUS26, palpography27 and lately IVUS-VH9. This latter concept is once again demonstrated in this study, where in 33.3% (7/21) of the patients IDTCFAs were present in at least two vessels. Moreover, in 31.7% of the vessels, two or more IDTCFAs were detected.
Spectral analysis of IVUS radiofrequency data has been used as a tool to assess plaque composition and remodelling11,12, since they are acquired in one single pull-back, simultaneous and complementary information can be retrieved. The previous manuscript from our group reporting the incidence of IDTCFA is essentially similar from the methodological point of view, but dramatically different from the quantitative point of view. So in the first report by Rodriguez-Granillo et al9, one of the criteria was the presence of NC>10% in a CSA with some NC in contact with the lumen, without considering its distribution along the luminal circumference (spotty or confluent). Thus, the main difference with the approach used in this report is the fact that the CSAs were included only if the necrotic core was confluent and this pool of NC represented more than 10% of the tissue present in the CSA and was in direct contact with the lumen. This different methodology may impact on the reported incidence of vulnerable plaque, but it offers a definition which is closer to the pathological one. Nevertheless, the inability to precisely measure the thickness of the fibrous cap makes our observations a surrogate of the pathological TCFA.
Positive remodelling has been associated with an unstable clinical presentation17,28 and with characteristic pathological6 and IVUS-VH11 lesion types. In line with these previous reports, the remodelling index found in this study shows the outward growth of these types of plaques. Some lipid lowering trials using conventional IVUS have suggested that there is a change in “composition” after a period of treatment29, whilst another with a similar design reported a change in the remodelling index18. IVUS-VH offers the opportunity to follow-up these lesions and to correlate longitudinally and simultaneously the composition and the change in the remodelling index.
It seems that among IDTCFAs there is different degree of disease. Not all of them have positive remodelling. The only opportunity to know if there is a different degree of risk among the same type of lesion is to look back into the pathological studies to learn which TCFAs have been undergone rupture. It is clear that ruptured plaque have more necrotic core, macrophage infiltration, calcification, less smooth muscles cells and more positive remodelling than TCFAs30. We can then conclude that those IDTCFAs having abundant necrotic core with positive remodelling have a higher risk, and it turns out that in this study these are few in number.
The clinical implications of these findings are manifold. First, the capability of simultaneously assessing more than one of the different acknowledged features of “high-risk” plaques could potentially enhance the prognostic value of the invasive detection of vulnerable plaque. Second, this combined assessment can potentially identify very high-risk plaques, thereby lowering the number of vulnerable plaques that deserve to be followed and ultimately treated.
Limitations
Although we have included all consecutive patients with three vessel IVUS assessment in a defined period of time, the sample size is small.
In general, the more severely diseased part of the vessel was stented before IVUS acquisition eliminating the analysis of potentially the most pathological region of interest.
The inferior axial resolution of IVUS-VH in comparison to histology remains a major handicap, but is partially compensated by the higher sampling rate of the ultrasonic approach when compared to the pathologic.
Conclusions
Cross sectional areas with necrotic core in contact with the lumen had a worse morphological profile than the ones without NC in contact with the lumen. The simultaneous assessment of IDTCFA and remodelling index identifies a reduced number of high-risk plaques. The findings of this study have potentially important clinical implications, since for the first time it sheds light into the possibility to follow these plaques after pharmacological treatment and/or to treat them locally.

