Abstract
Aims: To evaluate clinical outcomes at 24 months in an unselected population with ST-segment elevation myocardial infarction (STEMI) undergoing percutaneous coronary intervention (PCI) with drug eluting stents (DES), as compared with bare metal stents (BMS).
Methods and results: We report our series of 245 consecutive patients with STEMI undergoing PCI with DES (n=117) or BMS (n=128) from January 2003 to August 2005. The primary endpoint was the incidence of major adverse cardiac events (MACE) assessed at 24 months. The secondary endpoint was the incidence of stent thrombosis according to the Academic Research Consortium classification. Propensity score was used to adjust groups for baseline and angiographic characteristics. The adjusted rate of the primary end-point was lower in DES as compared with BMS group (15.2% vs 25.7%; HR 0.6 [95% CI 0.3-1.1], p=0.1), driven by a significant reduction of TVR (10.1% vs 23.0%; HR 0.4 [95% CI 0.2-1.0], p=0.03), without any significant difference regarding the composite end point of death and recurrent myocardial infarction (7.6% vs 5.4%; HR 1.4 [95% CI 0.4-5.0], p=0.6). The cumulative incidence of stent thrombosis was 5.3% in DES vs 0.8% in BMS group (p=0.06).
Conclusions: The use of DES in STEMI was associated with lower TVR rates, but no differences were observed about death and myocardial infarction at 24 months, as compared with BMS.
Introduction
Primary percutaneous coronary intervention (PCI) is considered the optimal approach to the management of myocardial infarction with ST-segment elevation (STEMI) when the procedure is performed expeditiously and by an experienced team1,2.
Drug eluting stents (DES) have been shown to reduce the risks of both restenosis and target-vessel revascularisation (TVR) after elective PCI, as compared with bare metal stents (BMS)3-6.
Moreover, data from recent trials7,9, registries10-12 and meta-analysis13, indicate that DES can be used safely in the setting of primary PCI and are likely to reduce the need for repeated revascularisation, as compared with BMS. In these studies, compared to BMS, DES were not associated with an increased risk of stent thrombosis (ST) at one year of follow-up. Nevertheless, recent reports from randomised trials and observational studies using historical controls have suggested that DES may be associated with increased rates of late ST, not observed in the first series of studies14-16. However, there is a widespread controversy13,17-18 regarding the actual incremental risk of ST and the clinical significance of this event associated with DES. Angioplasty in the setting of acute coronary syndromes (ACS) could theoretically predispose to ST19, but data are not all concordant7-9,20-23. To date, there are only a few data on the long-term outcome of the use of DES in patients with STEMI, which represent a high-risk subset of the real world. So we evaluated the 2-year clinical outcomes, and in particular the incidence of late and very late ST, of a series of consecutive patients with STEMI treated with DES or BMS.
Methods
Patients population
A total of 117 patients with STEMI underwent successful implantation of sirolimus-eluting stents (SES, Cypher; Johnson & Johnson-Cordis unit, Cordis Europe NV, Belgium) and paclitaxel-eluting stents (PES, Taxus stent, Boston Scientific Corp., Natick, MA, USA) between January 2003 and August 2005. A control group for comparison was composed of 128 consecutive patients with STEMI treated with conventional BMS in the same period.
Patients were eligible for the study if their symptoms began less than 12 hours before catheterisation and if the electrocardiogram showed new ST-segment elevation (greater than 0.1 mV, in 2 contiguous leads, or new or presumably new LBBB on the presenting ECG)24. Patients in cardiogenic shock (defined as systolic blood pressure persistently < 90 mmHg or the need for inotropic support or intra-aortic balloon pump implantation to maintain a blood pressure > 90 mmHg with evidence of organ end failure and increased left ventricular filling pressures) were included. Patients with an estimated life expectancy of less than 12 months and/or age > 75 years were excluded from the present analysis.
This protocol complied with Declaration of Helsinki and was approved by hospital ethic committee. Written consent form was obtained from every patient.
Procedures and post intervention treatments
Interventional procedures were performed according to international guidelines25-26 and the interventional strategy was left to the discretion of the operator, who was advised to use DES in patients with high risk factors for restenosis such as diabetes, small vessel, long lesion, bifurcation.
Baseline and post-procedural flows were evaluated according to the Thrombolysis In Myocardial Infarction (TIMI) criteria27. We administered oral aspirin (at a dose of 100 to 500 mg) and clopidogrel (300 mg) when patients first arrived at the hospital. Periprocedural administration of glycoprotein IIb/IIIa inhibitors was at the operator discretion and unfractioned heparin was administered to maintain an activated clotting time of > 250 seconds. Patients were advised to maintain lifelong aspirin therapy. Clopidogrel 75 mg/day or ticlopidine 250 mg twice daily were prescribed for one month in patients treated with BMS and for 6 or 12 months in patients treated with DES, depending on the complexity of the procedure.
Study endpoints and definitions
The primary endpoint was the incidence of cumulative major adverse cardiac events (MACE), assessed at 24 months, defined as the composite of death, recurrent myocardial infarction (MI) and TVR, either percutaneous or surgical.
Recurrent MI was diagnosed by recurrent symptoms and/or new electrocardiographic changes in association with increases in creatine kinase MB levels of > 1.5 times the previous value, if within 48 hours, or > 3 times the upper normal limit, if 48 hours after the index infarction28,29. TVR was defined as a reintervention driven by any lesion located in the same coronary artery and included CABG involving the infarct-related artery8.
The secondary endpoint was the incidence of ST defined by the angiographic documentation of either vessel occlusion or thrombus formation within, or adjacent to, the stented segment. According to the Academic Research Consortium (ARC) classification30, ST was defined, basing on elapsed time since stent implantation, as early (during the first 30 days), late (from 30 up until 360 days) and very late (after 360 days). Moreover, ST was claimed as definite (symptoms suggestive of an acute coronary syndrome and angiographic or pathologic confirmation of ST), probable (unexplained death within 30 days after the procedure or target vessel myocardial infarction without angiographic confirmation of ST), possible (any unexplained death after 30 days).
Information concerning in-hospital events was obtained from a centralised informatics database of our institution for those patients who stayed in our hospital and from the hospital records or by telephone contacts for those transferred to another hospital after the procedure.
The clinical follow-up data related to medications and clinical status were prospectively collected through scheduled outpatient clinic evaluations. Referring cardiologists, general practitioners and patients were contacted whenever necessary for further information. All repeated coronary intervention (surgical and percutaneous) and re-hospitalisation data were prospectively collected during follow-up using the centralised informatics system of our institution or contacting directly the hospitals were the patients were admitted or referred. All events were adjudicated by an independent, blinded endpoints committee.
Statistical analysis
Continuous variables were presented as mean±standard deviations, and were compared using Student unpaired t test. Categorical variables were presented as counts and percentages and compared with the chi-square test when appropriate (expected frequency > 5). Otherwise, Fisher exact test was used. A two-sided p value of less than 0.05 was considered to indicate statistical significance. Survival free of adverse events was estimated using Kaplan-Meier method and differences between curves were evaluated by log-rank test.
Propensity score matching31 was used to adjust groups for baseline and procedural characteristics. The matching variables were age, chronic renal failure, diabetes, ejection fraction, stent overlap, bifurcation lesion, presence of thrombus, reference vessel diameter, lesion length and residual dissection. The selection of the variables was based on a plausible association with ST19 and was made so as to get the best discriminating model as assessed by the Hosmer-Lemeshow goodness-of-fit statistic. All data were processed using the Statistical Package for Social Sciences, version 15 (SPSS, Chicago, IL, USA).
Results
Baseline and procedural characteristics
Baseline characteristics were comparable, except for a lower incidence of multivessel disease in patients treated with BMS (Table 1).
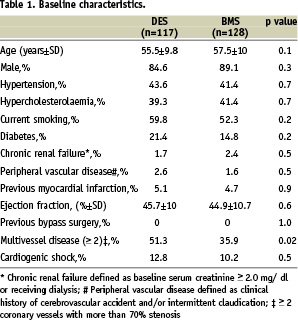
Lesion and procedural characteristics revealed several differences between the two groups (Table 2).
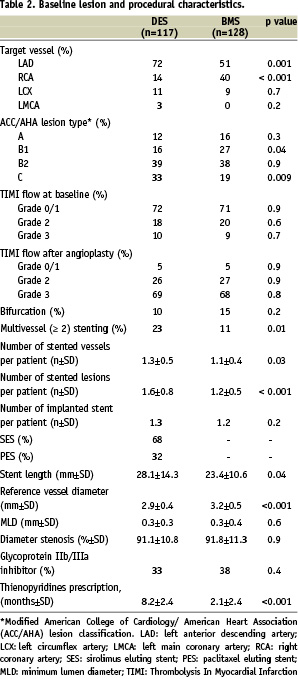
Of patients undergoing DES implantation, 68% received sirolimus eluting stent (SES) and 32% paclitaxel eluting stent (PES). Patients treated with DES had a higher frequency of left anterior descending lesions (p=0.001), whereas the right coronary artery tended to be the target vessel in patients treated with BMS (p < 0.001). Compared to the BMS group, the DES group had significantly smaller vessels (p < 0.001), longer stent length (p=0.04), type C lesions (p=0.009) and was more likely to receive multivessel and multilesion stenting (p=0.01 and p < 0.001, respectively). Glycoprotein IIb/IIIa receptor blockers were approximately used in one third of both groups (abciximab in all cases).
In-hospital outcomes
In-hospital outcomes are shown in Table 3.
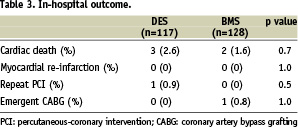
There were no significant differences between the two groups in the rates of cardiac death, MI, needing for repeat revascularisation by PCI or CABG.
Clinical outcome at 24 months
Clinical follow-up at 24 months was available in 97% of patients treated with DES and in 95% of patients treated with BMS.
As indicated in Table 4, the unadjusted clinical rates of mortality, cardiac mortality and MI were comparable between the two groups.
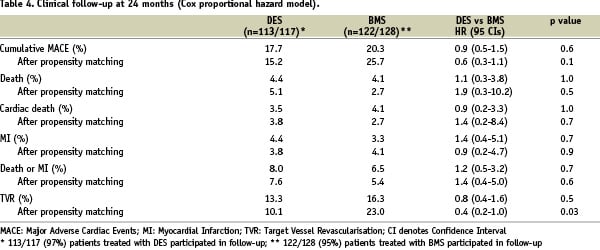
Of patients who received DES, 13.3% underwent TVR, while the respective rate for patients undergoing BMS implantation was 16.3%; this difference was not statistically significant (p=0.5), reflecting the increased risk of repeat revascularisation conferred by the complex coronary anatomy in DES patients. The composite endpoint of MACE was found in 17.7% of the DES group, almost comparable with the BMS group, in which a 20.3% incidence of MACE was found.
Notably, DES type was not a determinant of death (p=0.7), MI (p=1.0) or death/MI (p=0.7) when Cox analysis was performed in order to identify independent predictors of adverse events.
Of interest was the high incidence of ST (Table 5), which occurred in 5.3% of the DES patients (2.6% early ST, 0.9% late ST, 1.8% very late ST) compared with 0.8% in the BMS group (0% early ST, 0.8% late ST, 0% very late ST), showing a borderline significance (p=0.06).
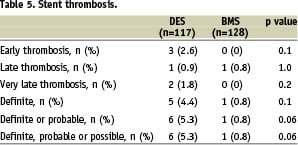
Of the total seven patients with definite or probable ST, one died, six presented with MI, and five patients were still on dual-antiplatelet therapy at the time of the event.
Propensity analysis was used to adjust for baseline and procedural imbalances between the study groups. The Hosmer-Lemeshow goodness-of-fit statistic for the adopted model was 0.99, indicating an excellent discrimination between treatments.
An equal number of patients treated with DES or BMS were matched on the basis of the similar propensity score, revealing no significant differences according to their demographic, clinical and procedural characteristics. Two-year cumulative incidence of the combined endpoint of death and re-AMI was 7.6% and 5.4% in the DES and BMS groups, respectively (HR 1.4 [95% CI, 0.4-5], p=0.6) (Figure 1A). No difference was apparent in terms of MACE (HR 0.6 [95% CI 0.3-1.1], p=0.1) (Figure 1B), but the use of DES was significantly superior to BMS in terms of TVR at two years (HR 0.4 [95% CI 0.2-1.0], p=0.03) (Figure 1C).
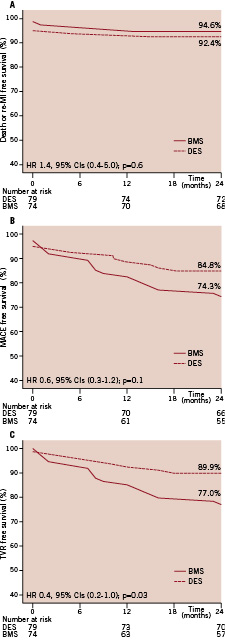
Figure 1. Propensity adjusted curves for survival free from death and myocardial infarction (A), major adverse cardiac events (B) and target vessel revascularisation (C) in patients treated with DES or BMS, respectively.
Discussion
The present study compared DES with BMS in the setting of primary PCI in a large single center unselected population. As compared with BMS, DES have demonstrated better outcome in reducing need for repeat revascularisation at two years, but no significant differences were observed in reducing death and MI.
There is a widespread controversy13,17-18 regarding the actual incremental risk of late ST and the clinical significance of this event associated with DES. In particular, whether the use of DES during AMI leads to an increased risk for the occurrence of late ST is unclear.
Recently, four randomised trials7-9,21 specifically studied the efficacy and safety of SES and PES in patients with AMI. In TYPHOON (Trial to Assess the Use of the Cypher Stent in Acute Myocardial Infarction Treatment With Balloon Angioplasty)7, the rate of repeated revascularisation procedures was significantly lower in SES group than in BMS control group, but no significant differences were observed in the rates of death, recurrent MI or in-stent thrombosis at one year. In PASSION (Paclitaxel-Eluting Stent versus Conventional Stent in Myocardial Infarction With ST-Segment Elevation) trial8, a non significant trend in favour of PES group was detected in the rate of cardiac death, recurrent myocardial infarction, TLR. The incidence of ST at one year was the same in both groups.
In SESAMI (randomised trial of Sirolimus-Eluting Stent versus bare-metal stent in Acute Myocardial Infarction) trial9, the use of SES reduced the incidence of restenosis, TLR and TVR but there were no statistical differences in the combined outcome of death and MI and in the incidence of stent thrombosis between the two groups at one year.
In STRATEGY (Single high-dose bolus TiRofiban And sirolimus-eluTing stent vs abciximab and Bare-Metal Stent in Myocardial Infarction) trial21, the cumulative incidence of death, myocardial infarction or TVR was lower in the tirofiban-SES compared with the abciximab-BMS group at two years but the rate of stent thrombosis did not differ. A different use of glycoprotein IIb/IIIa inhibitor may confound the interpretation of the comparison between the two types of stents.
Nevertheless, evidence for increased long term ST risk with DES is primarily from patient registries and not from randomised clinical trials, suggesting that the part of the incremental risk is associated with treatment of complex coronary lesions in real-world patients.
Moreover, an extended clinical follow-up appears mandatory to collect information on the long-term DES safety profile in STEMI patients, especially after thienopyridine therapy discontinuation.
Daemen at al22 completed a long term follow-up of a high risk subset of patients with STEMI, showing that the superiority of SES in decreasing TVR compared with BMS and PES at one year was no longer present at three years. In this study, ST occurred with an overall incidence of 2.4% and did not differ significantly across the treatment groups.
Our study is an observational prospective comparison between drug-eluting and bare-metal stents for PCI during acute myocardial infarction with ST-segment elevation at 24 months in the “real world”.
At the two year follow-up, the unadjusted cumulative incidence of the considered endpoints was similar in the two groups.
A trend towards higher rate of ST was noted in the DES group. In particular, there were six thrombosis in the DES and one thrombosis in the BMS group. This registry study obviously lacks statistical power to detect a difference between groups since ST is a rare event.
However, these data are consistent with previous randomised trials7-9,21 and registry reports22,23, comparing BMS and DES for primary angioplasty in acute MI, that found no difference in ST rate between the stent types. Of note, dual antiplatelet therapy was not able to prevent the early and late thrombotic events in DES group. The relative efficacy of dual antiplatelet therapy remains unknown. Further large dedicated studies are needed to answer this question definitively.
This study completed a 24 months follow-up of a real world complex unselected population. Its main limitation is the lack of a random assignment to treatment groups. In order to partly compensate for the baseline and angiographic imbalance between groups, we performed a propensity analysis. Nevertheless, despite our efforts to eliminate bias as much as possible, propensity score methods to adjust for this limitation is far from perfect.
Further, results are based on a relatively small patient cohort and therefore may have lack of statistical power, even if the population studied (245 patients) is quite large for a single centre registry.
Notably, in our registry, patients were selected on the basis of their clinical and angiographic risk for restenosis. The more complex angiographic characteristics of the DES group may justify the similar unadjusted MACE and TVR rates in both groups. After propensity score adjustment, in fact, we observed a clear trend in favour of DES about MACE, driven by a significant reduction in the need for TVR.
However, reduction of restenosis, as reported in main randomised trials, did not improve the prognosis: DES were not superior to BMS in reducing the incidence of hard endpoints such as death and re-AMI, also after propensity adjustment.
Therefore, based on these findings, the unlimited use of DES in the setting of STEMI may not be cost-effective32 and it seems appropriate to speculate that DES should be preferred to BMS only in the subset of patients with complex angiographic presentation or co-morbidities (e.g. diabetes) suggesting an unacceptable high risk of restenosis.

