Abstract
Aims: Most acute coronary syndromes are caused by plaque rupture. The risk of plaque rupture is related to plaque composition. The purpose of this study was to validate VH-IVUS for in vivo plaque characterisation.
Methods and results: Six rabbits were fed a cholesterol-supplemented diet for 12 to 18 months. Thereafter, VH-IVUS imaging of the aorta was performed. After sacrifice, the VH-IVUS images were matched to the corresponding histological cross sections. A total of 260 atherosclerotic plaques were analysed. VH-IVUS had a high sensitivity, specificity and positive predictive value for the detection of non-calcified thin cap fibroatheroma (88%, 96%, 87%, respectively) and calcified thin cap fibroatheroma (95%, 99%, 93%, respectively). These values were respectively 82%, 94%, 85% for non-calcified fibroatheroma and 78%, 98%, 84% for calcified fibroatheroma. The lowest values were obtained for pathological intimal thickening (74%, 92%, 70%, respectively). For all plaque types, VH-IVUS had a κ-value of 0.79. Linear regression analysis and Bland-Altman plots showed a strong correlation between VH-IVUS and histology for fibrous tissue, fibrofatty tissue, necrotic calcified tissue and confluent necrotic core.
Conclusions: VH-IVUS showed a good accuracy for in vivo plaque characterisation and is a promising technique for the detection of the vulnerable plaque.
Introduction
Most of the acute coronary syndromes are caused by rupture of atherosclerotic plaques. The pathogenesis of plaque rupture is complex, but is closely related to the plaque composition. Rupture-prone atherosclerotic plaques are characterised by a large necrotic core, covered by a thin fibrous cap and are called thin cap fibroatheroma (TCFA)1. In vivo characterisation of the plaque composition could allow the early detection of these high-risk lesions before plaque rupture has occurred.
Gray-scale intravascular ultrasound (IVUS) has only a limited value for the identification of plaque composition2. Analysis of the IVUS radiofrequency data provides a more accurate and reproducible technique for plaque characterisation3-5. Virtual histology (VH) is an IVUS-based method that uses spectral analysis of the backscattered radiofrequency signal to perform plaque characterisation3,6. VH-IVUS identifies four different plaque components (fibrous, fibrofatty, necrotic and calcified tissue) and has been reported to have a good accuracy for characterisation of these different tissue components7,8. However, validation studies of VH-IVUS for identification of the plaque type (i.e. pathological intimal thickening, non-calcified or calcified fibroatheroma, and non-calcified or calcified thin cap fibroatheroma) are scarce9.
In the present study, New Zealand white rabbits were fed a long-term cholesterol supplemented diet, resulting in the development of atherosclerotic plaques in the aorta that strongly resemble human atherosclerotic plaques. The aim of this study was to validate VH-IVUS for in vivo identification of the plaque type.
Materials and methods
IVUS imaging
Six New Zealand White rabbits (Merelbeke, Belgium) were fed a 0.3% cholesterol supplemented diet for 12 to 18 months to induce atherosclerotic plaques in the aorta. The rabbits were then anaesthetised with sodium pentobarbital (30mg/kg intravenously). The right femoral artery was dissected under sterile conditions and a 4 Fr sheath was introduced. Under fluoroscopy, a 0.014-inch angioplasty guidewire (Abbott Vascular Laboratories, IL, USA) was advanced into the aorta. A 20 MHz phased-array IVUS catheter (Eagle Eye™, Volcano Therapeutics Inc, Rancho Cordova, CA, USA) was positioned in the ascending part of the aortic arch. Under continuous ECG monitoring, the IVUS catheter was pulled back at a continuous speed of 0.5 mm/sec from the ascending part of the aortic arch to the bifurcation of the aorta into the iliac arteries.
All experiments were approved by the Ethical Committee of the University of Antwerp and conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
VH-IVUS analysis
All IVUS images were digitised and stored on the hard disk of the IVUS console (Volcano Therapeutics Inc, Rancho Cordova, CA, USA) for spectral analysis of the backscattered radiofrequency signals. VH-IVUS analysis gives a different colour code to fibrous (green), fibrofatty (yellow green), necrotic (red) and calcified (white) tissue. The predicted plaque composition is presented as a colour coded tissue map. The different tissue components were expressed as percentage of total plaque area (= intimal + medial area)10,11.
Histology
After VH-IVUS imaging, the animals were euthanised by an overdose of pentobarbital. The ventral side and all side branches of the aorta were marked with indelible ink (Bradley Products Inc, Bloomington, MN, USA). After fixation in 4% formaldehyde (pH 7.4), the aorta was cut into 2 mm rings. The tissue was then dehydrated overnight and embedded in paraffin. Serial 5 µm cross-sections were stained with haematoxylin-eosin, Masson trichrome and Movat pentachrome. The following definitions for the different tissue components were used. Fibrous tissue was characterised by bundles of collagen fibres with little or no lipid accumulation. Areas of loosely packed collagen bundles with interspersed extracellular lipid were labelled as fibrofatty tissue. Necrotic core was defined as a hypocellular plaque cavity devoid of collagen and containing necrotic debris and cholesterol clefts. Calcified tissue was defined as compact calcium crystals, characterised by an intense staining on haematoxylin-eosin staining. All images were analysed using a colour image analysis system (Image Pro Plus 4.1, Media Cybernetics Inc, Silver Spring, MD, USA). The different tissue components were expressed as percentage of total plaque area.
Correlation between histology and VH-IVUS for different tissue components
The VH-IVUS frame corresponding to every tissue section was identified by matching of the side-branches on VH-IVUS and histology. The origin of the side-branches and the position of the black ink were used for orientation of the IVUS-images and the histological slides, respectively. The percent area of fibrous tissue, fibrofatty tissue, necrotic calcified tissue and confluent necrotic core was compared between histology and VH-IVUS.
Identification of plaque type by VH-IVUS
To determine the accuracy of VH-IVUS for identification of the plaque type, every tissue section was compared to three consecutive frames of VH-IVUS. The following definitions for classification of atherosclerotic plaques by histology and VH-IVUS were used12,13: (1) pathological intimal thickening (PIT) was defined by histology and VH-IVUS as a mixture of mainly fibrous and fibrofatty tissue, with limited amounts of microcalcifications (< 10% of plaque volume) and necrotic core (< 10% of plaque volume); (2) fibroatheroma (FA) was defined by histology as a confluent necrotic core (> 10% of plaque volume), covered by a thick (> 150 µm) fibrous cap and by VH-IVUS as a confluent necrotic core (> 10% of plaque volume) with an overlying fibrous cap on three consecutive frames; (3) thin cap fibroatheroma (TCFA) was defined by histology as a confluent necrotic core (> 10% of plaque volume), covered by a thin (< 150 µm) fibrous cap and by VH-IVUS as a confluent necrotic core (> 10% of plaque volume) without an overlying fibrous cap (in direct contact with the lumen) on three consecutive frames. If a FA or TCFA contained more than 10% dense calcium, they were defined as calcified FA or calcified TCFA, respectively.
Intra-observer and inter-observer variability
Manual contour detection of the intimal and medial-adventitial boundaries was performed by two independent observers (inter-observer variability). One of the observers also performed an analysis of the boundaries after a three weeks interval to determine the intra-observer variability.
Statistical analysis
For analysis of sensitivity, specificity and positive predictive value, histology was used as ‘gold standard’. The κ-value was calculated to determine the degree of agreement between histology and VH-IVUS14. Correlation between histology and virtual histology for the percent area of every tissue component (fibrous, fibrofatty, necrotic calcified tissue and confluent necrotic core) was evaluated by linear regression analysis and Bland-Altman plots. These plots were constructed by plotting the difference in percent area of the different tissue components between histology and VH-IVUS versus the average percent area15. Linear regression analysis and Bland-Altman analysis were also performed for intra- and inter-observer variability. Statistical analysis was performed by using SPSS (v14, Chicago, IL, USA). A p value < 0.05 was considered significant.
Results
Histology and VH-IVUS strongly correlate for the different tissue components
Linear regression analysis showed a strong correlation between histology and VH-IVUS for the percent area of fibrous tissue (R=0.81, p<0.001), fibrofatty tissue (R=0.70, p<0.001), necrotic calcified tissue (R=0.73, p < 0.001) and confluent necrotic core (R=0.65, p < 0.001) (Figure 1).
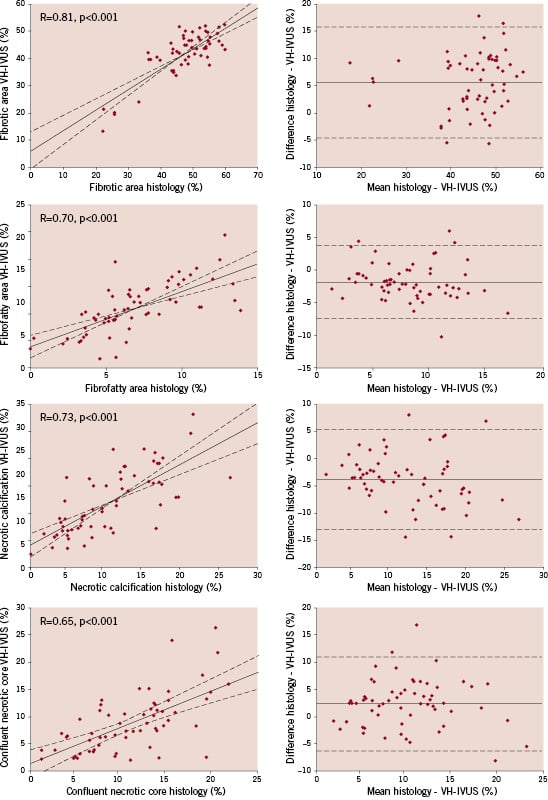
Figure 1. Correlation between histological and VH-IVUS data for fibrous, fibrofatty, necrotic calcified tissue and confluent necrotic core. Left, the linear regression line shows a strong correlation between histology and VH-IVUS for the different tissue components. The dashed lines represent the 95% confidence limits. Right, the corresponding Bland-Altman plots are shown. The difference between histology and VH-IVUS did not vary in any systematic way over the range of measurements. The solid line denotes the mean difference, the dashed lines represent the 95% confidence limits.
Bland-Altman analysis showed a mean difference (± 2 SD) in percent area of 5.9 (± 10.2)% for fibrous tissue, –1.7 (± 5.5)% for fibrofatty tissue, –3.5 (± 9.2)% for necrotic calcified tissue and 2.3 (± 8.6)% for confluent necrotic core. Furthermore, the Bland-Altman plots demonstrated that the differences between histology and VH-IVUS did not vary in any systematic way over the range of measurements for fibrous tissue, fibrofatty tissue, necrotic calcified tissue and confluent necrotic core (Figure 1).
VH-IVUS is a highly sensitive and specific method for identification of TCFA
A total of 260 atherosclerotic plaques were analysed. The sensitivity, specificity and positive predictive value for the different plaque types are summarised in Table 1.
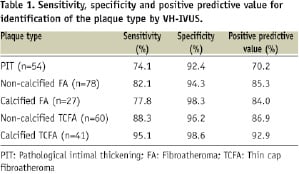
VH-IVUS had a sensitivity of 88% and 95% for the detection of non-calcified and calcified TCFA, respectively. TCFA were correctly detected by VH-IVUS as a necrotic or necrotic-calcified core in direct contact with the lumen, without an overlying fibrous cap (Figure 2).
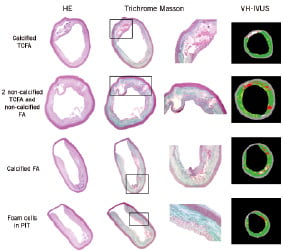
Figure 2. Haematoxylin-eosin (HE) and Masson trichrome staining of different histological types of atherosclerotic plaques. A magnification of the rectangle in the Masson staining is also shown. In the right-hand column the corresponding VH-IVUS image is presented. The images show that VH-IVUS correctly identifies a calcified thin cap fibroatheroma (TCFA), two non-calcified TCFA and a non-calcified fibroatheroma (FA), and a calcified FA. At the bottom, an accumulation of foam cells in a pathological intimal thickening (PIT) is misclassified by VH-IVUS as a necrotic core.
VH-IVUS misclassified 12% of the non-calcified TCFA (7/60) and 5% of the calcified TCFA (2/41) as PIT or FA (Table 2).
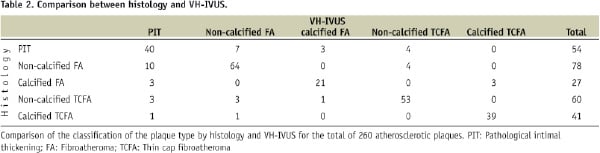
VH-IVUS correctly identified 82% of the non-calcified FA and 78% of the calcified FA as a necrotic or necrotic-calcified core, covered by overlying fibrous tissue. VH-IVUS misdiagnosed 5% of non-calcified FA (4/78) and 11% of calcified FA (3/27) as TCFA, indicating that the fibrous cap thickness was underestimated by VH-IVUS.
For the detection of PIT, VH-IVUS showed a sensitivity of 74.1% and a positive predictive value of 70.2%. These low values can be partially explained by the presence of large accumulations of foam cells, which were frequently misclassified by VH-IVUS as necrotic or calcified tissue (Figure 2).
The κ-value for all plaque types was 0.79, indicating a good agreement between histology and VH-IVUS.
Intra-observer and inter-observer variability
The intra-observer and inter-observer variability of the intimal boundaries and the medial-adventitial boundaries were low. For intra-observer variability, Bland-Altman analysis showed a mean difference (± 2 SD) of –0.10 (± 0.82) mm2 for the luminal area and –0.13 (± 2.14) mm2 for the vessel area (Figure 3).
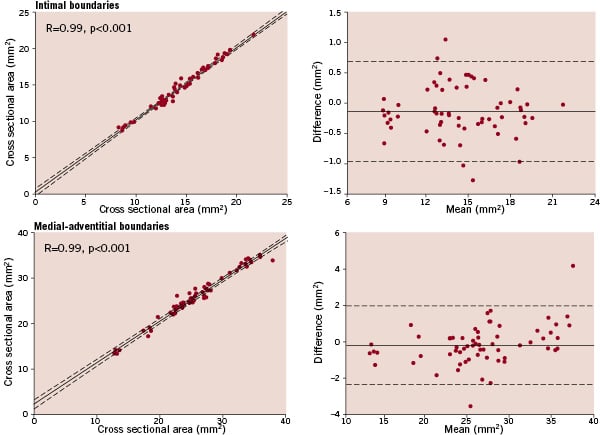
Figure 3. Intra-observer variability of intimal and medial-adventitial boundaries. Left, linear regression analysis shows a strong correlation between two measurements, which were performed by one observer with a 3 weeks interval. The dashed lines represent the 95% confidence limits. Right, the corresponding Bland-Altman plots are shown, which were constructed by plotting the difference between the two measurements versus the mean of both measurements. The solid line denotes the mean difference, the dashed lines represent the 95% confidence limits.
For inter-observer variability, Bland-Altman analysis showed a mean difference (± 2 SD) of 0.12 (± 1.12) mm2 for the luminal area and 0.39 (± 3.05) mm2 for the vessel area (Figure 4).
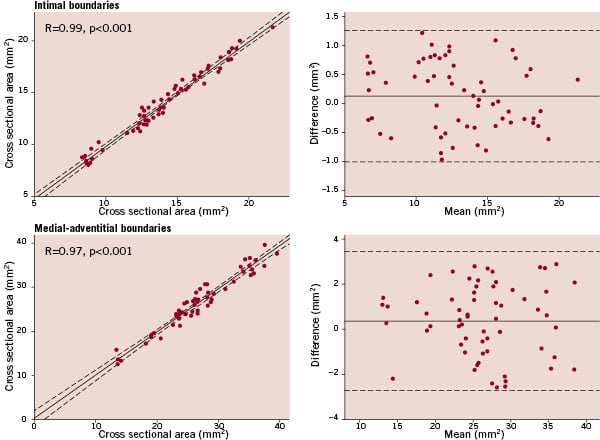
Figure 4. Inter-observer variability of intimal and medial-adventitial boundaries. Left, linear regression analysis shows a strong correlation between two measurements, which were performed by two observers. The dashed lines represent the 95% confidence limits. Right, the corresponding Bland-Altman plots are shown, which were constructed by plotting the difference between the two measurements versus the mean of both measurements. The solid line denotes the mean difference, the dashed lines represent the 95% confidence limits.
Discussion
The present study showed a high accuracy of VH-IVUS for in vivo identification of the plaque type in a rabbit model of atherosclerosis. Atherosclerotic plaques were divided into five different types: pathological intimal thickening, calcified and non-calcified FA, and calcified and non-calcified TCFA. Because IVUS has an axial resolution of about 100-150 µm, the fibrous cap can only be detected if it is thicker than 150 µm. The fibrous cap thickness was therefore used to differentiate FA (> 150 µm) and TCFA (< 150 µm). The VH-IVUS image of a TCFA is a necrotic core in direct contact with the lumen, without an overlying fibrous cap. Conversely, a FA is presented by VH-IVUS as a necrotic core, covered by an overlying fibrous cap. VH-IVUS had a high sensitivity, specificity and positive predictive value for the detection of calcified and non-calcified TCFA. VH-IVUS also showed a good sensitivity for the detection of calcified and non-calcified FA. The lowest values of sensitivity and specificity were obtained for pathological intimal thickening, which can be partially explained by the presence of large accumulations of foam cells in the atherosclerotic plaques. These foam cells, which are not only found in atherosclerotic plaques of animal models16,17, but also in human atherosclerotic plaques18, were misclassified by VH-IVUS as necrotic or calcified tissue, leading to an incorrect determination of the plaque type. A widely used parameter to assess the accuracy of a diagnostic tool is the κ-value. The κ-value is a measure of agreement and ranges from 0 (low agreement) to 1 (perfect agreement). For all plaque types, VH-IVUS had a κ-value of 0.79, which indicates that VH-IVUS has a good accuracy for in vivo identification of the plaque type.
Recently, Granada et al19 reported an accuracy of 38–58% for plaque characterisation in porcine coronary arteries. The reasons for this low accuracy are unclear, but could be related to the high incidence of early fibroproliferative lesions, which do not resemble the advanced human atherosclerotic plaques that were used for the development of VH-IVUS. In our study, long-term feeding (12 to 18 months) of a 0.3% cholesterol-supplemented diet to rabbits resulted in atherosclerotic plaques with strong similarities to advanced human atherosclerotic plaques, including the presence of large necrotic cores.
Linear regression analysis and Bland-Altman plots showed a good correlation between histology and VH-IVUS for fibrotic, fibrofatty and necrotic calcified tissue, which is in accordance with previous studies7,8. There was also a strong correlation between histology and VH-IVUS for the percent area of the confluent necrotic core. The Bland-Altman plots showed that the standard deviations of the differences between histology and VH-IVUS for the different tissue components are large. The reasons for this large variability are unknown, but could be related to artefacts induced by fixation and histological processing. However, it is also possible that the exact plaque composition (e.g. exact size of the necrotic core) cannot be reliably predicted by VH-IVUS and that the reported percent area of the different tissue components should be interpreted carefully. Importantly, a recent study of Rodriguez-Granillo et al20 showed that VH-IVUS measurements of the plaque composition are reproducible, which indicates that longitudinal studies, examining the effect of interventions, are possible.
VH-IVUS has several limitations, including a limited axial resolution. Our definition of VH-IVUS derived TCFA is different from the histologically defined TCFA with a critical fibrous cap thickness of 65 µm. The absence of a fibrous cap therefore implies that the fibrous cap is < 150 µm thick, and thus may not truly be ‘pathologically thin’. At this moment the exact value of VH-IVUS derived TCFA for predicting future cardiovascular events is unknown. This question is currently being investigated in PROSPECT (Providing Regional Observation to Study Predictors of Events in the Coronary Tree) trial (http://clinicaltrials.gov/ct2/show/ NCT00180466), which will analyse the relationship between a specific plaque type according to VH-IVUS and the risk of future acute coronary syndromes.
Another limitation is the inability of VH-IVUS to identify early or organised thrombi. A recent study showed that the presence of thrombi reduced the accuracy of VH-IVUS21. This shortcoming of VH-IVUS had no influence on our results because the atherosclerotic plaques in the rabbit aorta do not spontaneously rupture.
Despite these limitations, VH-IVUS seems to be a promising technique for in vivo plaque characterisation. Our results show that VH-IVUS is accurate for the identification of the plaque type, especially of TCFA. VH-IVUS can therefore be used for the early detection of the vulnerable plaque.
Acknowledgements
The authors are indebted to Rita Van den Bossche and Cor Van Hove for their excellent technical assistance.
Funding
Jozef Van Herck is a research assistant of the Fund for Scientific Research (FWO, Flanders).

