Abstract
Aim: To investigate in terms of clinical, haemodynamic and biochemical profile the safety and efficacy of the Impella Recover® LP 2.5 left ventricular assist device during elective high risk percutaneous coronary interventions (HR-PCI).
Methods and results: Ten out of twelve patients were initially enrolled to receive PCI supported by the Impella catheter; eight underwent pressure-volume (PV) loop analysis while one patient was monitored by intra-cardiac echocardiographic. Free haemoglobin (fHb), B-type natriuretic pepetide, catecholamines, aldosterone, angiotensin II, and endothelin were assessed before, every 40 minutes as average during the procedure and at 3, 12, 24 and 48 hours after intervention. The Impella catheter was used for 144±88 min [median (IQR) 108 (85-198)], and was removed immediately after the procedure in all but one patients. In 6, 3 and 2 patients, fHb levels increased above 1, 5 and 10 times the upper limit of normal (ULN), respectively. No significant effect was found on the tested biomarkers in Impella-supported procedures. The PV analysis showed the occurrence of an acute volume increase in the majority of patients immediately after Impella insertion that tended to persist even at maximal pump speed. This was confirmed by the intracardiac echocardiography that was performed in one patient.
Conclusions: Our data, although preliminary due to the limited sample size, does not encourage the routine use of Impella Recover® LP 2.5 in HR-PCI. Additional studies are required to confirm and elucidate the mechanisms responsible for the acute LV volume loading and to quantify the degree of haemolysis induced by the pump in a broader set of patients.
Introduction
The need for haemodynamic support in high risk percutaneous interventions (HR-PCI) remains widely debated and controversial. It is well recognized that elective HR-PCI can be safely performed without percutaneous cardiopulmonary support or on intra-aortic balloon pump (IABP) in most circumstances. However, in patients with a borderline haemodynamic status, ongoing ischaemia or cardiogenic shock, insertion of an IABP just before coronary instrumentation has been associated with improved outcomes1,2.
In view of the lack of prospective randomized data, the recently published ACC/AHA/SCAI PCI guidelines recommend the use of cardiopulmonary support for patients only at the extreme end of the spectrum of haemodynamic compromise, such as those patients with extremely depressed left ventricular function or patients with cardiogenic shock3. Therefore the decision to proceed with IABP or other devices before percutaneous coronary intervention (PCI) remains a clinical judgement made by the physician based on the high-risk characteristics of coronary anatomy and the overall status of the patient3-5. The limited effectiveness of actual support and device-related complications have so far hampered a more widespread use of these devices in our daily practice.
The Impella Recover® LP 2.5 left ventricular assist device (LVAD) (ABIOMED® - Impella CardioSystems GmbH, Aachen, Germany) is a catheter-based miniaturized rotary blood pump (4 mm -12Fr- in outer diameter), that is placed retrograde through the aortic valve and aspirates blood from the LV cavity and expels it in the ascending aorta. Under clinical conditions the pump provides up to 2.5 L/min at its maximal rotational speed. The device is mounted on a 9 Fr pigtail catheter, giving the advantage of percutaneous insertion via a 13 Fr femoral sheath (www.impella.com). We recently reported the case of a single patient with severe LV impairment receiving an Impella-supported coronary intervention. Twelve patients have been considered subsequently for Impella-supported elective HR-PCI as part of a single-centre, investigator-driven protocol. Our complete results, in terms of clinical, haemodynamic and biochemical findings, are presented here.
Methods
Patients
Appropriate candidates for this clinical investigation were adult patients of either gender with stable or unstable angina pectoris and a clinical indication for percutaneous coronary revascularisation. In addition, the patients had to have at least one coronary artery stenosis that was amenable to angioplasty as well as left ventricular ejection fraction (LVEF) < 30%, or an angioplasty target vessel supplying > 50% of the viable myocardium, or combined left main and right coronary revascularization or complex lesion(s) in the last remaining patent artery, or refusal of surgical standby because of contraindications to cardiopulmonary bypass. All patients in this study provided written informed consent. The protocol received approval by our local Ethics Committee on Human Research.
Haemodynamic measurements
A 7 Fr combined pressure-conductance catheter was introduced before positioning of the Impella via the left femoral artery and placed along the long axis of the LV. The catheter was connected to a Cardiac Function Lab (CD Leycom, Zoetermeer, the Netherlands) for online display and acquisition of LV pressure-volume loops. The conductance catheter was calibrated using thermodilution and hypertonic saline dilution, as previously described6. Cardiac output was measured by thermodilution catheter (COtd) and by multiplying stroke volume as measured by conductance catheter by heart rate (COlv). The difference between COlv and COtd is mainly due to activation of the Impella pump. The baseline (T0) haemodynamic data was acquired subsequently. Immediately after Impella catheter positioning the minimum level of pump speed [i.e. number of rotations per minutes (rpm)] expected to compensate the spontaneous backflow inside the pump cannula based on the actual pressure gradient between the aorta and LV during diastole was set and all haemodynamic measurements were repeated (T1). The same acquisition scheme was finally performed (T2) after setting the pump at its maximum level of activation (i.e. 50/52,000 rpm), unless suction was detected at console inspection.
Since pump flow is linearly related to motor current, suction was identified as a reduction in motor current at constant level of pump activation. In such cases, the highest pump speed at which no suction occurred was selected for the T2 measurements. In all cases, technical assistance provided by the producing company (ABIOMED® - Impella CardioSystems GmbH, Aachen, Germany) was available to check for the correct positioning and functioning of the Impella device. After obtaining haemodynamic measurements at different levels of pump activation, the procedure was started with active Impella support. In one patient (#8) all haemodynamic measurements were performed at the end of intervention while the baseline (T0) was obtained immediately after Impella catheter removal.
Intracardiac echocardiographic examination
One patient in whom no pressure-conductance catheter was introduced, underwent intracardiac echocardiographic examination through the AcuNav catheter (Siemens Corp. Germany) inserted in the right ventricle. After obtaining a stable short axis view at the mitral valve papillary muscles level, the baseline LV area was quantified. After Impella catheter insertion, the same echocardiographic measurements were repeated at 35,000, 43,000 and 50,000 rpm. Special care was taken to ensure a stable position of the AcuNav catheter during the whole acquisition process.
Biochemical measurements
Free haemoglobin (fHb): Whole blood was centrifuged, plasma and buffy coat removed and red cell layer washed several times with saline (pH: 7.30) until the supernatant was negative for protein. The resting solution was centrifuged at 1650xg. The stock haemoglobin solution was prepared by diluting the supernatant with Sørensen phosphate buffer (pH: 7.40; M/15) to a concentration of 30 mg per 100 ml. The exact concentration was measured by a photometer that had been standardized for haemoglobin by iron and oxygen-capacity determinants, as previously described7. The upper limit of normal reference for fHb was 10 µmol/L.
Biomarkers: Blood (2-3 ml) was collected in chilled heparinized tubes containing 3 mg. of glutathione and centrifuged within 15 min at 4°C (15 min, 3000xg). Plasma was stored at –70°C. Determination of catecholamines was done by HPLC with fluorimetric detection.8 Blood for BNP, endothelin and aldosterone measurement was collected in EDTA-tubes; the plasma was stored at –70°C after preparation. BNP was measured by a commercially available immunoradiometric method (Shionoria, Osaka, Japan). Endothelin was measured using a QuantiGlo immunoassay kit from R&D Systems, Abingdon, UK. Aldosterone was measured with a commercially available radioimmunoassay kit (Coat-A-Count, Diagnostic Products Corporation, Los Angeles, CA, USA). For angiotensin II determination blood was collected in chilled tubes containing an inhibitor solution of EDTA, Remikiren and Lisinopril. Determination was performed by Seppak extraction and radioimmunoassay as previously described.9
Statistical analysis
Values are expressed as mean±SD or median and interquartile range (IQR) as appropriate. The effect of Impella-supported intervention on the parameters of interest (i.e. haemodynamic variables and biochemical measurements) was investigated by the use of ANOVA for repeated measures and Post hoc comparisons were performed by Tukey’s Honest Significance Difference test where appropriate. All statistical tests were 2-tailed. Probability was considered significant at a level of <0.05. Statistical analysis was performed using Statistica 6.1 Software (Statsoft Inc.).
Results
12 patients were enrolled in the protocol from June 2004 to April 2005. In Patient#4, the sheath of the Impella catheter could not be introduced due to an iatrogenic dissection of the left femoral artery which occurred during arterial access. Similarly, patient#7 underwent treatment without Impella support due to unavailability of the LVAD catheter at the time of the procedure. Thus, 10 patients undergoing intervention supported by the Impella catheter were finally included in the present study. Their baseline and procedural characteristics are summarized in Tables 1 and 2.
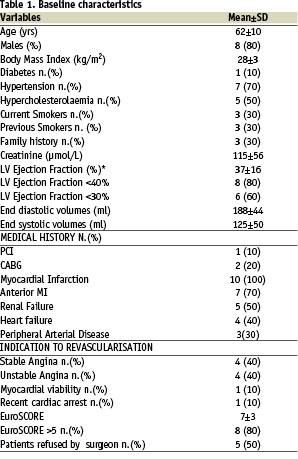
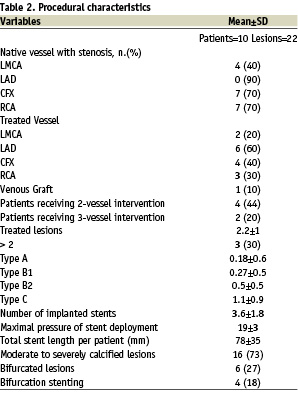
The overall average left ventricular ejection fraction (LVEF) was below 40%, while LVEF was less than 30% in 6 patients. All patients had experienced a previous myocardial infarction that had involved the anterior wall in 7 patients. Four patients (40%) had a history of symptomatic heart failure, eight (80%) were at high surgical risk according to the EuroSCORE and 5 (50%) patients had been previously refused for cardiac surgery. One patient (#8) with an occluded right coronary artery underwent treatment for a diffusely diseased venous-jump graft anastomosed to the left anterior descending and circumflex arteries, while all others received treatment of the native coronary system, including two patients who underwent treatment for an unprotected distal left main stenosis. On average, there were 2.2 lesions per patient, the majority of which were C-type according to the modified AHA lesions classifications; 3.6 stents per patient were implanted with a total stent length of more than 75 mm.
The positioning of the Impella was uncomplicated in all patients except two, in whom a second attempt to re-position the catheter was needed due to difficulties in removing the guide-wire after the initial positioning of the Impella catheter.
The duration of LVAD support was 144±88 min (median (range) [IQR]: 108 (60-352) [85-198]); in one patient the Impella was left in place after coronary intervention, while in the other 9 patients it was removed at the end of the procedure.
In patient #8, while receiving intervention in the only remaining open vessel, the arterial pressure remained above 90 mmHg even during a transient (approximately 25 seconds) episode of no-reflow which followed stent expansion (Figure 1).
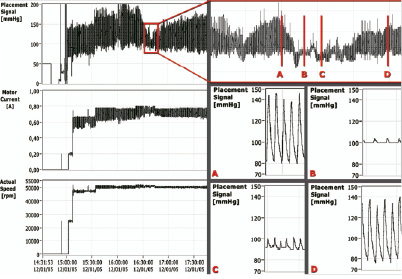
Figure 1. Aortic pressure (mmHg) signal recorded by a micromanometer positioned near the outlet of the Impella catheter), used for catheter positioning and Impella actual motor current (A) and speed (rpm) during intervention in patient#8. In the top right corner, a magnification of the aortic pressure at the time of a no-reflow phenomenon while treating a graft lesion is shown. Panel A and D: Aortic pressure before and after the occurrence of no-reflow phenomenon. Panel B and C: Aortic pressure during no-reflow phenomenon showing almost absent (especially in figure 2) systo-diastolic excursions as expression of transient depression of left ventricular pumping activity.
In half of the patients, the maximum level of pump activation (i.e. around 50,000 rpm) could not be maintained during the procedure due to the presence of a suction effect that disappeared in all cases after decreasing the rotational speed to approximately 40,000 rpm.
Groin haemostasis was obtained by manual compression with/without Femostop® in 4 patients, with 10 french Prostar XL 10® in 2 patients, with a single 8 french Angioseal® in 2 patients, with two Angioseals in parallel (one 6 french and one 8 french) in one patient, and through a surgical percutaneous closure in one case. Three patients developed major groin haematoma at the site of Impella insertion. Four patients required red blood cells transfusion of two or more units due to substantial blood loss during and/or after the procedure.
Impact of Impella on haemolysis
Levels of fHb before, during and after PCI assisted by the Impella catheter are shown in Figure 2.
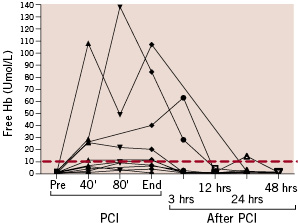
Figure 2. Free haemoglobin (Hb) levels in relation to timing of intervention (PCI). The red line represents the upper limit of normality of the tested parameter according to the employed photometer test.
Overall, 6 (60%) patients showed fHb levels beyond the upper limit of normal (red line), while 5 patients had persistent fHb elevation in two or more consecutive samples. In the patient in whom the Impella was left in place after the procedure, the fHb peak occurred at the end of the procedure and not at the time of Impella removal. Similarly, in the patient who showed the highest levels of haemolysis, the peak of fHb (approximately 14 times the upper limit of normal) occurred 30 minutes before Impella removal. On the other hand, in one patient, fHb peaked 3 hours after removal of the Impella. In all of the other patients (n=7) fHb level peaked at the time of Impella removal.
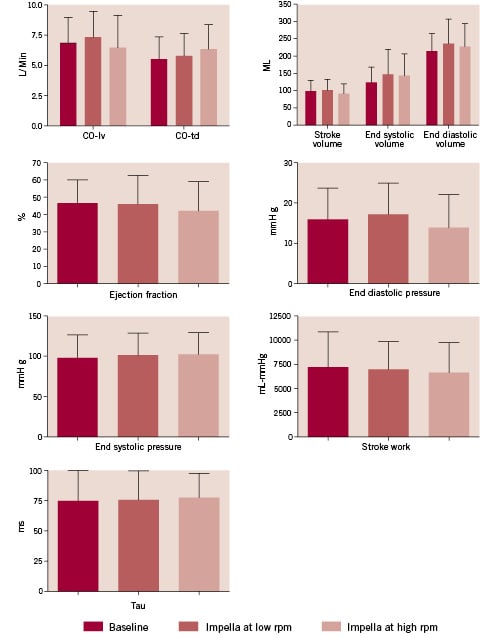
Figure 3. Haemodynamic variables recorded through the conductance catheter or the thermodilution catheter according to Fick’s technique before (baseline), or when the Impella catheter in place during a minimal or maximal level of activation (rpm). COtd: systemic cardiac output measured by thermodilution catheter; COlv: cardiac output according to left ventricle stroke volume as measured by the conductance catheter.
Haemodynamic effects of the Impella pump
In the first consecutive nine patients enrolled a 7-Fr combined pressure-conductance catheter was introduced via the left femoral artery and placed along the long axis of the left ventricle directly before positioning of the Impella catheter (8 patients) or immediately after intervention (1 patient, #8). Pressure recordings were judged to be of high quality in all patients included, while in one case (#11) volume-based parameters had to be discarded due to the presence of multiple and diffuse artefacts. There was no significant effect on any of the studied haemodynamic parameters with regards to Impella pump insertion and activation except for end diastolic pressure (p<0.01), which showed a slight increase at T1 (17±9 mmHg vs. 15±8 mmHg at T0) followed by a decrease soon after the Impella was maximally activated (13±8 mmHg at T2). The left ventricular cardiac output, stroke volume, end-systolic and end-diastolic volumes) tended to increase when the Impella catheter was inserted and activated at low rpm at T1 -this level of activation was expected to compensate backflow inside the cannula caused by the pressure gradient between aorta and left ventricle during diastole-, followed by an opposite trend as soon as the pump reached the highest possible rpm at T2.
Ejection fraction and systemic cardiac output measured by the thermodilution catheter tended to decrease and increase when going from baseline (T0) to T1 and to T2, respectively, while end-systolic pressure and relaxation time constant tau remained practically unchanged over time. A patient-by-patient analysis based on actual pressure-volume loop analysis is shown in Figure 4.
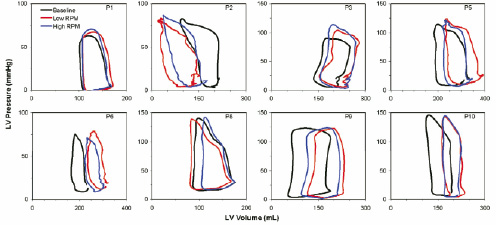
Figure 4. Individual pressure-volume loops at baseline (black line), immediately after positioning the Impella catheter at minimal level of activation (blue line), and with Impella at maximal speed (red line).
In the majority of the patients (#1, #3, #5, #6, #9 and #10) there was a sudden (detectable immediately after catheter insertion) increase in left ventricular volumes and, in some patients in the end-diastolic pressure as well, as soon as the Impella pump was inserted and activated at low rpm.
The maximal activation of the pump (T2) produced an opposite effect, with variable degrees of LV volume unloading as compared to T1. In all these individual cases, however, a volume increase compared to baseline was still noted at T2. In patient #2 a progressive volume unloading going from baseline to T1 and from T1 to T2 was evident, while patient#8 showed an intermediate pattern of response, with minor volume overload at T1 that was overcompensated with respect to baseline at T2.
Thus the heterogeneity of response to Impella activation may well explain the absence of a net significant haemodynamic effect produced by the pump compared to baseline when all data were pooled.
Effect of Impella-supported procedure on circulating biomarkers
The change over time of the tested biomarkers in patients undergoing intervention supported by the Impella catheter as compared to baseline is shown in Figure 5.
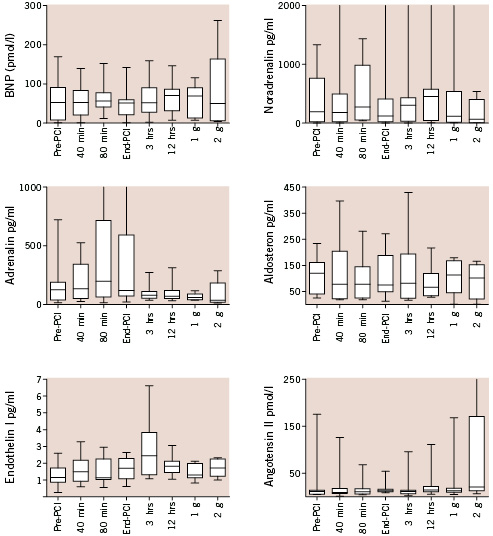
Figure 5. Levels of tested biomarkers in relation to the timing of intervention. None of the studied parameters were significantly affected by the Impella-supported intervention at the analysis of variance. BNP: B-type natriuretic peptide (g=day).
None of the selected parameters showed a significant change over time, despite clear individual heterogeneity in response to the treatment as revealed by the increase in data dispersion (i.e. data range and interquartile range) during and after intervention with respect to baseline.
Effect of Impella-supported procedure on left ventricular volumes based on the intracardiac AcuNav echocardiographic examination
In the last patient (#12), the effect of the Impella support on LV volumes was investigated by an intracardiac echocardiographic AcuNav catheter examination. The results are given in Table 3 while figure 6 shows four left ventricle short axis views at baseline (A, B) and after activating the Impella support at 43,000 rpm during diastolic and systolic phases, respectively (C, D).
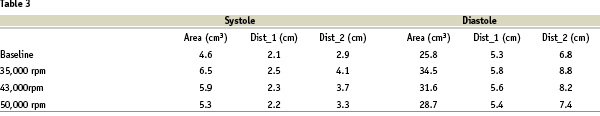
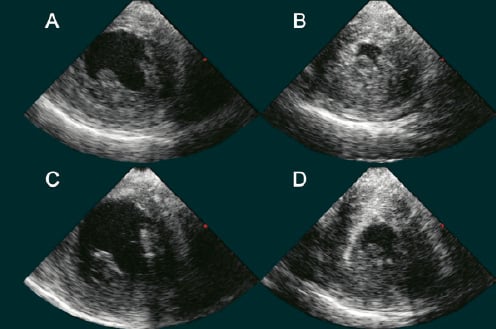
Figure 6. Effect of Impella support on LV volumes in patient#12, investigated by intracardiac echocardiographic AcuNav catheter. Left ventricle short axis views during diastolic and systolic phases at baseline (A, B) and after activating the Impella support at 43000 rpm,(C, D).
Discussion
The Impella LVAD Recover® LP 2.5 is a miniaturized catheter-mounted rotary blood pump that is placed through the aortic valve, aspirates blood from LV cavity, and expels it in the ascending aorta. In clinical conditions the pump provides up to 2.5 L/min at its maximal rotational speed of 50/52,000 rpm. If placed without activation, the catheter would per se induce some backflow inside the cannula due to the pressure gradient during the diastolic phase between aorta and left ventricle. The actual amount of flow shunting back to the LV chamber during each diastole and the level of pump activation that is necessary to antagonize this intra-cannula insufficiency is dependent on the actual pressure gradient. Based on in vitro tests, a level of activation around 30,000 rpm is expected to neutralize the effective backflow (with some minor leak during diastole followed by some minor positive output during the systole) at a typical diastolic gradient between the aorta and the LV of 60/70 mmHg.
According to this principle and guided by an engineer who had a major role in developing the Impella device, we set the level of pump activation at T1 in order to have no net flow within the pump cannula during each cardiac cycle. We thus expected to have a comparable haemodynamic profile at T1 as compared to baseline.
However our findings indicate that after Impella positioning and with minimal activation at T1, a substantial volume overload occurred with respect to baseline in 7 out of 8 patients studied, with only one individual able to (over) compensate for the initial volume increase during maximal activation of the pump at T2. In the remaining case, a clear LV volume and pressure unloading at each step of Impella catheter activation was noted. Thus, in only 2 out of the 8 patients who underwent pressure-volume analysis in the present protocol, a final LV unloading was detected at T2.
These observations, coupled with the rather limited sample size of the study may explain the observed overall neutral effect of the Impella pump on almost all studied haemodynamic variables and biomarkers, including B-Type natriuretic peptide, whose production and secretion being linked to LV wall stretch, is known to closely reflect the haemodynamic status of the heart.
It could be argued that the simultaneous positioning of 12 Fr (Impella LVAD) and 7 Fr (pressure-conductance) catheters through the aortic valve might theoretically cause significant aortic regurgitation by disturbing adequate valve closure. However a pattern of volume overload was confirmed also by intracardiac echocardiographic examination, during which only the Impella catheter was positioned through aortic valve. Potentially, transseptal positioning of the conductance catheter might be desirable in future investigations. Thus although we cannot fully rule out the possibility that the two catheters in parallel might have increased the magnitude of volume overload due to increased aortic regurgitation, we believe this does not explain our current findings.
Our study focused on elective patients undergoing HR-PCI, who had had their medical treatment optimized. As a consequence, all patients had a normal or near-normal LV end-diastolic pressure before intervention and this may have contributed to the increase of the magnitude of volume regurgitation and LV overload during the diastolic phases of the cardiac cycle at T1. This may also explain the high incidence of suction effect observed in our study. In patients with acute haemodynamic compromise or during the acute phase of ischaemia (such as the transient no-flow phenomenon observed in patient#8) the LV diastolic pressures may be, or become, particularly elevated, thus limiting the degree of volume regurgitation towards the LV, while favouring a forward output towards the aortic arch. As an example, in patient#8, a clear effect of the Impella pump, was observed at the stage in which a dramatic no-flow phenomenon occurred during intervention in the last remaining patent artery. As shown in Figure 1, arterial pressure remained constantly above 90 mmHg while LV function was extremely, although transiently, depressed as shown by the minor systo-diastolic excursion. Indeed the patient remained completely asymptomatic during this phase and no inotropic support was needed.
At this point the reasons behind the immediate volume overloading which followed Impella pump positioning in most cases may only be speculative since our protocol was not designed to investigate this issue. A possible explanation is that while blood is continuously expelled into the aorta from the outlet of the Impella catheter, blood is at the same time regurgitating back into LV due to aortic valve insufficiency (peri-cannula leak). This may be the consequence of the interplay between two factors: 1) The presence of the Impella catheter (4 mm in outer diameter) may interfere with aortic valve closure, particularly when the pump is far from being perpendicular with respect to the aortic valve plane in patients with severely calcified leaflets; 2) in cases where the Impella pump is placed too deeply inside the LV although positioning may appear satisfactory, based on pressure tracing inspection at the device console, the outlet orifices may remain too closely in contact with the aortic valve plane. When the blood is expelled with great velocity out into the ascending aorta, turbulent vortices may be created that may force the aortic valves to open. In this situation, the higher the degree of pump activation, the higher might be the induced aortic regurgitation. This may explain why despite maximal activation of the pump in 6 patients out of 8 studied, the device could not compensate the acute LV volume overload observed at T1.
If our speculations are correct, the haemodynamic effects provided by the Impella catheter depended a great deal on the haemodynamic status of the patient but may also be affected by the actual position of the Impella catheter. Future studies, potentially including transseptal placement of the conductance catheter and slow pull-back of the Impella device after initial positioning, may be needed to substantiate our speculations. Concomitant, transoesophageal echocardiographic examination may provide additional insight.
Finally, two issues deserve attention: a) the occurrence of haemolysis as a consequence of pump activation and b) bleeding at the site of pump insertion.
a) We observed occurrence of haemolysis in 6 out of 10 patients. In five, this was detected in two or more samples collected around 40 minutes apart. In three cases, levels of fHb increased more than 5 times the upper limit of normal (ULN) while in two individuals 10 times the ULN was found. At the same time, we clearly could observe that the degree of observed haemolysis was not strictly related to the duration of pump activation. This may be explained by the fact that the relatively older erythrocytes in the circulating pool may be subject to injury first. Thus after this early peak of haemolysis, the degree of fHb elevation may remain controlled even if the pump remains activated for several hours. Our study could not confirm this hypothesis, which needs to be tested in future investigations. The presence of suction during pump activation would be theoretically able to increase the occurrence of haemolysis due to increase shear stress. However, fHb levels at peak was not different in those with as compared to patients without suction during intervention, which makes the possibility that suction was the explanation for the high rate of haemolysis observed in our series quite unlikely.
b) The need to insert an arterial 13 Fr sheath before Impella pump insertion exposes the patient to a risk of bleeding at the site of puncture, as witnessed by our experience. We believe that echo-guided puncture of the common femoral artery followed by 10 Fr Prostar XL 10® insertion is the best approach in order to ensure effective and safe vascular access when the Impella pump needs to be inserted.
Study limitations
Our study involved a limited number of patients and no control group was used. According to our protocol, the patient at baseline was supposed to be the internal reference of the study. However this may carry important limitations especially in interpreting our results in terms of biomarkers, which are known to be affected by the coronary intervention itself.
The need to place two catheters simultaneously in place through the aortic valve to obtain PV loop signal might limit the external validity of our findings; transseptal placement of the conductance catheter would be preferable for future studies to avoid interference with aortic valve closure system while the impella catheter is in place.
Conclusions
Our findings on the safety and efficacy of the Impella Recover® LP 2.5 during HR-PCI, although preliminary due to the limited sample size, cannot support its routine use in this setting. Additional studies are required to confirm and investigate the mechanisms of the acute LV volume overload observed in the majority of the cases enrolled in the present study. Whether and how much the acute LV volume overload affects the haemodynamic support provided by the Impella pump remains unclear based on our data. Similarly, a better quantification of the degree of pump-induced haemolysis in a broader set of patients is warranted.
Reviewer comments:
I agree with the statement, in the introduction of the previous case report (Cath and CV Interventions 2005; 65:263-267) as well as this manuscript, that the role of assist devices generally in high-risk PCI is limited and controversial. I believe that is the case because:
a) it is hard to predict which stable patients will ‘crash’; this was shown in the cardiopulmonary support registry experience (JACC 1993;21:590-596), and
b) cardiopulmonary support and IABP have significant potential for vascular adverse outcomes (CPRS also has haematologic and pulmonary physiologic effects), and perhaps most important
c) the time one takes inserting and setting up equipment, that neither the operator nor the lab crew use very frequently, can be spent getting the culprit vessel stented in a ‘crashing’ patient.
I don’t think there is any doubt the heart-lung bypass machine can support the entire cardiac output of a patient in full arrest (for example AJC 1989;64:967-970), but it takes a while to get the 12 and 14 F cannulate in place and most lab crews have limited or no regular experience setting it up, priming it, etc, etc.
The IABP provides some forward output augmentation, a little LV unloading and a lot of diastolic augmentation of coronary flow in patients in shock ( for example either Coronary Artery Disease 1991;2:649-660 or Circulation 2000;102:364-365). Most experienced operators have used the IABP enough to have it in place and operational in about a minute or 2, unless there is major vascular issue.

