Abstract
Timely detection of vulnerable atherosclerotic plaques is of the utmost importance to prevent acute clinical events, such as unstable angina, myocardial infarction and sudden death. Advanced plaques show a dense infiltration of macrophages, of which a subpopulation strongly expresses uncoupling protein 2 (UCP2). This protein uncouples adenosine triphosphate (ATP) production from mitochondrial respiration and thereby converts the loss of potential energy in heat production. In early atherosclerotic lesions, UCP2 seems to fulfil an atheroprotective effect by reducing reactive oxygen species (ROS) production and/or by inhibiting monocyte recruitment. Because the amount of macrophages in these lesions is limited, effects on plaque temperature cannot be detected. As the macrophage content of the plaque increases and the plaque progresses toward an unstable phenotype, ROS production is overwhelming, and possibly cannot be counteracted by the antioxidant properties of UCP2. However, the increasing number of UCP2-positive macrophages inside advanced plaques correlates with increased plaque temperature. This thermogenic effect can be detected by intravascular thermography and may be indicative of rupture-prone regions. Thus, UCP2 expression in macrophages in advanced plaques can be considered a biochemical link between plaque temperature and vulnerability.
Uncoupling proteins
The mitochondrion is a double membrane-enclosed organelle that converts organic materials into cellular energy. During mitochondrial respiration, a fixed number of protons are pumped by some components of the electron transport chain from the mitochondrial matrix into the intermembrane space, generating a proton gradient across the inner mitochondrial membrane. The electrochemical potential difference, created as such, drives the protons back into the matrix through adenosine triphosphate (ATP) synthase and provides energy for the conversion of ADP to ATP (Figure 1).
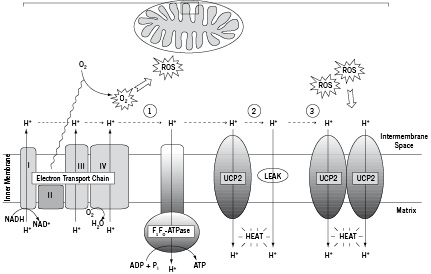
Figure 1. Uncoupling action of UCP2. The electron transport chain builds up a proton gradient across the inner mitochondrial membrane, which provides energy for the conversion of ADP to ATP through the ATP synthase (1). Besides this tightly coupled process protons leak back across the membrane using UCP2 or non-protein membrane pores (leak). This uncoupling dissipates the H+ gradient as heat (2). Incomplete oxygen reduction leads to production of reactive oxygen species (ROS) inducing cellular oxidative stress and damage. To protect the cell against oxidative stress, UCP2 is upregulated so that more protons can be redirected to the matrix. This event leads to increased heat production (3).
However, the active transport of protons from the mitochondrial matrix to the intermembrane space is not perfectly coupled with proton re-entry and ATP synthesis. Apparently, a portion of the created proton gradient is consumed by proton backflow to the matrix via non-protein membrane pores or protein/lipid interfaces, thereby ‘wasting’ energy that is derived from the oxidation of substrates (Figure 1)1-3. At least a part of this ‘proton leak’ is mediated by uncoupling proteins (UCPs)3. UCPs belong to the mitochondrial anion carrier gene family and, as suggested by their name, can uncouple ATP production from mitochondrial respiration which results in the loss of potential energy as heat4-6.
The term ‘uncoupling protein’ was originally used for the mitochondrial membrane protein thermogenin or UCP1, which is uniquely present in mitochondria of brown adipocytes that regulate body temperature in small rodents, hibernators and mammalian newborns. The UCPs are best characterised in mammals where six different orthologues have been identified. UCP2 is ubiquitous and highly expressed in the lymphoid system, macrophages, and pancreatic islets7, whereas UCP3 is predominantly expressed in skeletal muscle and the heart8. In this review, we will focus on recent findings from mammalian studies showing a potential role for UCP2 in atherogenesis and temperature heterogeneity of vulnerable atherosclerotic plaques.
UCP2: a multifunctional protein?
According to recent evidence, UCP2 in atherosclerosis seems to be involved in thermogenesis and handling of ROS through mitochondrial uncoupling, as explained below.
Thermogenic properties
Eleven of the twelve amino acid residues critical for UCP1 function are conserved in UCP29, suggesting that UCP1 and UCP2 share similar functions. Indeed, the elevated expression of UCP2 in ground squirrels during hibernation advocates a role for this protein in mammalian non-shivering thermoregulation10. Moreover, infrared thermography revealed thermogenesis after UCP2 overexpression in cultured cells11,12. Some claim that the latter results must be viewed with caution as overexpression of the UCP homologues may produce an aberrant form of uncoupling. This is probably caused by the improper insertion of the overexpressed protein into the mitochondrial inner membrane and, as such, the observed proton leak is non-specific and unregulated13. Furthermore, there has been much debate regarding the thermogenic capacity of UCPs other than UCP1, because UCP2 expression can be up to 1000-fold lower than that of UCP114. Therefore, the expected rate of proton conductance controlled by UCP2 would be much less than that produced by UCP1. However, this debate is focusing on thermogenesis as it pertains to core body temperature rather than energy dissipation in the form of heat at the mitochondrial level. The distinction between these is critical. It has been suggested that UCP2 is not thermogenic, because it does not appear to contribute to the generation of core body temperature15. Because UCP2 knock-out mice are not cold-sensitive and do not respond to a high fat diet, an implication of UCP2 in diet or cold induced thermogenesis remains controversial15. Nonetheless, UCP2-positive brain regions have a significantly higher local temperature when compared to other sites or to the core body temperature16, indicating that UCP2 may have micro-environmental thermogenic function, at least in the brain17.
Antioxidant properties
Mitochondria possess a mechanism called ‘mild’ uncoupling, which prevents large increases in the proton electrochemical gradient when ADP is not available18. This mechanism reduces the production of reactive oxygen species (ROS) by the respiratory chain, and modulates the ATP/ADP ratio3,6. UCP2 has an important role in the mediation of the ‘mild’ uncoupling mechanism and hence in the control of ROS. If ROS overwhelm the oxidant defence of the cell (i.e. a state of oxidative stress), damage to cellular macromolecules such as lipids, protein and DNA may occur19. As a consequence, control of ROS production by UCP2 underlies a putative protective role against oxidative damage as observed for example in vulnerable atherosclerotic plaques20.
UCP2 and atherosclerosis
Atherosclerosis is a chronic disorder of the arterial intima, most notably in large-and medium-sized vessels, and slowly progresses over a period of decades before clinical symptoms become manifest21-24. During plaque development, monocytes are recruited from the circulation in the subendothelial space, where they differentiate into macrophages25. These recruited macrophages endocytose modified lipids via scavenger receptors to form foam cells. The death of macrophage-derived foam cells by necrosis leads to the formation of a necrotic core, rich in cholesterol and cell debris22. Importantly, ROS promote atherogenesis via several important enzyme systems, including xanthine oxidase, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, and inducible nitric oxide synthase (iNOS)26. The production of ROS by monocytes/macrophages, as main cellular component of the plaque, is at least in part regulated by UCP2 under various stress conditions15,27,28. Indeed, transient overexpression of UCP2 in monocytes reduces both steady-state levels of intracellular ROS and the production of ROS in response to H2O2, MCP-1, and TNF-α29. Similarly, UCP2 overexpression in human aortic endothelial cells reduces ROS generation and leads to a significant increase in eNOS and decrease in endothelin-1 mRNA expression30.
Beside its involvement in ROS production, overexpression of UCP2 in monocytes decreases transendothelial migration of circulating monocytes and prevents excessive accumulation of monocytes/ macrophages in the arterial wall, thereby reducing atherosclerotic plaque formation29. In contrast, lack of UCP2 in monocytes from UCP2 knock-out mice accelerates atherosclerotic plaque development and induces a macrophage rich but collagen-poor plaque phenotype20. Moreover, the common 866G/A single nucleotide polymorphism in the human UCP2 promoter yields stronger transcriptional activity in monocytes and endothelial cells and associates with asymptomatic carotid atherosclerosis in women31,32. All these findings suggest a protective role for UCP2 in early atherosclerosis.
Macrophage-rich plaques are prone to rupture, so their timely detection and optimised treatment is necessary to prevent acute clinical events. Currently, there is no single technique that can conclusively identify a vulnerable plaque33. The benefit of locating individual vulnerable plaques in a multifocal disease is even a topic of intense debate33. Yet, detecting isolated (unifocal) vulnerable plaques can be valuable for a number of reasons. For example, some people rely on invasive treatment due to failure of pharmacological treatment. Indeed, effects of pharmacological agents can take a while to become effective, in which period it might be necessary to detect the individual plaques, perhaps to even treat them invasively. In view of the prediction and prevention of cardiovascular events, there is now significant interest in developing new ways to identify vulnerable plaques by non-invasive means, such as magnetic resonance imaging (MRI) and computed tomography (CT), as well as by invasive means, such as ultrasound (and the related techniques of integrated backscatter and elastography), near-infrared spectroscopy, angioscopy, optical coherence tomography, and thermography33. Below, we will focus on thermography of atherosclerotic plaques in relation to UCP2 expression.
Thermography of atherosclerotic plaques
Several ex vivo and in vivo studies showed a correlation between inflammation and heat production both in experimental and human atherosclerotic plaques34-38, albeit a large variability in the temperature differences has been observed. This variation is mainly due to different measuring techniques and the heterogeneity of the lesions. Temperature differences are mainly measured with thermistor probes or basket catheters (thermocouple-based catheters made of a nitinol system with small and flexible thermocouples, resolution 0.001 °C, accuracy 0.02 °C)36 and increase significantly in plaques from patients with unstable angina and acute myocardial infarction as compared to patients with stable angina or age-matched controls without plaques34. In human carotid artery plaques or plaques from cholesterol-fed rabbits, plaque temperature correlates positively with macrophage density and plaque thickness, but inversely with the distance of the macrophage clusters from the luminal surface35,37. Moreover, there is an association between increased plaque temperature and acute phase reactants such as C-reactive protein and serum amyloid A, indicating that inflammation is of importance for heat production in the atherosclerotic plaque38. Decreasing the macrophage content of atherosclerotic plaques without altering plaque area, either by dietary cholesterol withdrawal or by administration of statins, promotes a reduction in temperature heterogeneity37,39,40. Taken together, a relationship exists between macrophage-rich (unstable) atherosclerotic plaques and the temperature differences present in these plaques. If vulnerable plaques in coronary arteries can be detected by the generation of thermal maps, it would be possible to identify patients at risk for an acute coronary syndrome. Once identified, these patients can be treated earlier, resulting in risk reduction of acute coronary events.
UCP2 expression in vulnerable atherosclerotic plaques and its relation with temperature heterogeneity
Advanced plaques show a dense infiltration of macrophages of which a subpopulation strongly expresses UCP2 protein. Laser capture micro-dissection experiments confirmed that these macrophages express abundant UCP2 mRNA41. In contrast, smooth muscle cells (SMC) in early lesions (adaptive intimal thickening) and normal media do not express UCP2 (only weak expression of UCP3). The UCP2-positive macrophages in advanced atherosclerotic plaques are associated with fatty acid accumulation and signs of increased oxidative stress such as oxidised lipids, iNOS and nitrotyrosine12,41. Moreover, UCP2-positive macrophages are localised predominantly in the subendothelial layer of advanced plaques, which is clinically relevant because thermography mainly measures the temperature of the superficial cell layers. Macrophages in deeper layers of the plaque do not overexpress UCP2 and therefore do not seem to influence temperature heterogeneity41.
In early atherosclerotic lesions, UCP2 seems to fulfil an atheroprotective effect by reducing ROS production and/or by inhibiting monocyte recruitment, as explained above. Because the amount of macrophages in these lesions is limited, effects on plaque temperature due to UCP2-mediated proton translocation cannot be detected. As the macrophage content of the plaque increases and the plaque progresses toward an unstable phenotype, ROS production is overwhelming, and possibly cannot be counteracted by the antioxidant properties of UCP2. However, the increasing number of UCP2-positive macrophages inside advanced plaques correlates with increased plaque temperature. This thermogenic effect can be detected by intravascular thermography and may be indicative of rupture-prone regions (Figure 2).
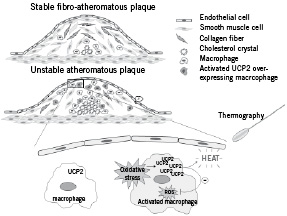
Figure 2. Schematic representation of a stable and an unstable atherosclerotic plaque and the biochemical link with UCP2. A stable plaque consists of a thick fibrous cap containing smooth muscle cells and collagen fibres and has only a small necrotic core and few monocytes/macrophages. An unstable plaque, on the other hand, is characterised by a relatively large lipid core, a high macrophage density in the shoulder region and a thin fibrous cap. In response to oxidative stress macrophages in unstable plaques stimulate the expression of UCP2 to prevent an excess of free radicals. The translocation of H+ through UCP2 generates heat that can be detected via thermography and may be an indicator of a vulnerable plaque.
Possibly, the observed heat production is a side-effect rather than the main function of UCP2 and may have substantial consequences for the structure of the plaque. Heating increases selective macrophage apoptosis, probably by inactivation of NF-κB and melting of oxidised cholesterol crystals42. Although some consider these effects as beneficial, the free cholesterol and apoptotic debris are not adequately removed in advanced lesions43. As a consequence, upregulation of UCP2 in advanced plaques may stimulate inflammatory responses and enlargement of the necrotic core and may ultimately lead to plaque rupture. Thus, UCP2 expression in macrophages in advanced plaques can be considered a biochemical link between plaque temperature and vulnerability.
Acknowledgements
Research was supported by the fund for Scientific Research (FWO)-Flanders (project N° G.0308.04 and G.0113.06), the University of Antwerp (NOI-BOF) and the Bekales Foundation. Wim Martinet is a postdoctoral fellow of the FWO-Flanders.

