Abstract
Aim: To evaluate whether contrast enhanced MRA (CE-MRA), including a dedicated MRA coil and a high dose of contrast material, correlates to i.a. digital subtraction angiography (DSA) when used for treatment planning in patients with peripheral arterial obstructive disease (PAOD).
Methods and results: A retrospective CE-MRA and i.a. DSA comparison was used to evaluate 2200 vessel segments with stenosis or occlusion in the pelvic or lower extremity arteries in 100 patients. A 1.5T MR unit (Magnetom Symphony Quantum, Siemens) employing a peripheral angiography array surface coil and automatic table movement was used. The interventional approach was planned according to both CE-MRA and DSA findings. Visual material was blinded and reviewed by two experienced radiologists.
In 98.95% (Observer 1) and 98.1% (Observer 2) CE-MRA revealed an exact correlation of the grade as well as length of stenosis compared to DSA. The sensitivity was 100% and 95.3% for observers 1 and 2 and its specificity 98.3% and 100%, respectively. The interobserver agreement between was 0.98 and 0.96 for observers 1 and 2, respectively. Suggested treatment and interventional approach based on the CE-MRA findings corresponded to DSA in 97%.
Conclusion: CE-MRA is an excellent modality for treatment planning in patients with PAOD. Especially in patients with restenosis or reocclusion, CE-MRA enables an accurate, non-invasive diagnosis and facilitates treatment planning.
Introduction
Peripheral arterial occlusive disease (PAOD) is a significant source of morbidity in western society, with an estimated incidence of 2 to 10 new cases per 1000 persons per year1.
Treatment options consist of exercise training, modification of cardiovascular risk factors and percutaneous or surgical intervention. Before intervention, precise mapping of the location and extent of PAOD is necessary. For this purpose, duplex ultrasound (US) is routinely used in many centers and enables reasonably accurate detection of stenosis or occlusion in the aorto-iliac and femoro-popliteal arteries2. However, duplex US is operator-dependent and time-consuming. Additionally, obese patients or patients with excessive bowel gas or calcified arteries are difficult to examine3,4.
Intra-arterial digital subtraction angiography (i.a. DSA) remains the gold standard for comprehensive examination of the peripheral arteries5,6 even though it is time-consuming, costly and exposes the patient to ionising radiation and potentially nephrotoxic iodinated contrast agents7,8. Additionally, i.a. DSA is associated with complications such as dissection, bleeding and other puncture related complications.
Since the introduction of magnetic resonance angiography (MRA), there have been promising developments and innovations in the technique. Especially the development of the ultra-fast three dimensional gradient echo sequences (3D-GRE), the application of paramagnetic contrast agents as well as technical advances such as dedicated peripheral surface array coils and the moving-bed technique have opened new opportunities for the diagnostic use of MRA in the pelvic and lower extremity arteries9-11. With contrast enhanced magnetic resonance angiography (CE-MRA), images similar to those generated by i.a. DSA can be obtained from the infrarenal aorta to the ankles12,13.
Though the advantages of CE-MRA have been described in previous studies, mostly with small patient numbers12,13, the utility of CE-MRA as a tool for interventional planning has not been investigated extensively. The aim of the present study was to evaluate whether CE-MRA, including a dedicated MRA coil and a high dose single volume contrast material, correlates with i.a. digital subtraction in treatment planning for patients with PAOD.
Material and methods
Patients
Between January 2003 and December 2003 CE-MRA was employed in 615 patients with PAOD in our department. 100 of these patients meeting the following criteria were selected for retrospective analysis:
– had received peripheral i.a. DSA from the renal arteries down to the feet within 2-4 weeks before or after CE-MRA.
– had received a CE-MRA examination from the infrarenal aorta down to the feet employing dedicated peripheral MRA coils, the moving table technique as well as 1.0 molar Gadobutrol as high dose contrast agent (weight adjusted <70kg=15 ml; >70kg=20ml) as i.v. contrast material.
The retrospective analysis was approved by our institutional review board. All patients gave written informed consent for both the CE-MRA examination as well as the i.a. DSA.
CE-MRA was performed either prior to intervention for treatment planning, or as a follow-up examination after treatment of one side and combination of treatment planning for the contralateral side.
CE-MRA
CE-MRA was performed on a 1.5 Tesla MR unit (Magnetom Symphony Quantum, Siemens AG, Erlangen) using the moving-table technique and a peripheral angiography array surface coil.
The examination was performed with the patient in a supine, feet-first position. To adequately position the pelvic and lower extremity arteries into a horizontal line, foam cushions to fix knees, lower legs and feet were used.
For lower extremity imaging from the feet up to the inguinal ligament, a peripheral array surface coil was used. For pelvic area imaging a body array surface coil was added.
To optimize the time for contrast application the Care Bolus method with semiautomatic bolus detection in the infrarenal aorta was employed. The application of the paramagnetic contrast agent was performed with an automatic injection system (Medrad) via an 18-21 Gauge access in the cubital vein. We used i.v. bolus application of a single body weight adjusted dose of contrast agent (Gadobutrol (Gadovist®), Schering AG, Berlin) (Table 1).

The peripheral vascular system of the pelvic and lower extremity was divided into three fields of view (FOV):
Field of view I: abdominal aorta and pelvic arteries
Field of view II: infrainguinal arteries and popliteal arteries
Field of view III: arteries below the knee to feet
To localize the infrarenal aorta for the Care Bolus and the other major vessels in the three fields of view, scout images with two dimensional sequences in axial, sagittal and coronal slice orientation were acquired. On this basis the definite planning of the MRA sequences was made. The plain images were acquired beginning with FOV I and FOV II, in each case followed by an automatic table translation of 390 mm towards the head, and completed with FOV III, followed by an automatic translation of 780 mm towards the feet into the original starting position. After applying the contrast material, the contrast enhanced images were acquired in the same manner. The acquisition time for FOV I and II was 23 seconds each, and for FOV III 26 seconds plus 11 seconds of delay for each table translation.
Finally, the plain images were subtracted from the contrast enhanced MRA images and the maximum intensity projection (MIP) images of all three fields was reconstructed in a 180° rotational view with a single MIP-image every 15°.
i.a. DSA
Procedures were performed in the interventional radiology suite with employment of the AXIOM Multistar angiographic unit (Siemens). After local anesthesia in the right or left groin using 10 ml Scandicain 1% (Astra Zeneca, Germany) the common femoral artery was punctured and a 5 French (F) sheath (Terumo Europe) was introduced. A 5F pigtail catheter (Cordis Endovascular) was placed at the level of the renal arteries to perform the i.a. DSA of the abdominal aorta. The pigtail catheter was then placed just above the aortic bifurcation followed by the i.a. DSA of the pelvic and peripheral arteries using i.a. DSA with step-translation technique.
Contrast material was applied in bolus technique with the application of iodixanol (Visipaque 320 mg I/mL [Amersham Health]) via an automated power injector and the following amount and flow rates: abdominal aorta: 30 ml, flow rate 15 ml/sec.; pelvic and peripheral arteries: 80 ml, flow rate 10 ml/sec.
A selective i.a. DSA of the ipsilateral side was performed via the sheath and/or of the contralateral side via cross-over placement of a5 F multipurpose catheter (Cordis Endovascular) in all cases with angiographically proven lesions in the region of the calf.
Data analysis
All images were blinded and evaluated by two experienced interventional radiologists. CE-MRA and i.a. DSA images were read separately with a time interval of 2 weeks between the two readings. Treatment recommendation was established for both imaging modalities.
Vessels evaluated were: the common iliac artery, the external iliac artery, the internal iliac artery, the common femoral artery, the deep femoral artery, the superficial femoral artery, the popliteal artery, the tibio-fibular trunk, the anterior tibial artery, the fibular artery, and the posterior tibial artery. All 11 peripheral vessels from the pelvic arteries to the lower leg were evaluated separately for each side. In cases where there was more than one lesion within a vessel we presumed that the lesion with the most severe pathology was the one with the greatest hemodynamic effect.
The findings were graded into five categories as normal, stenotic (<50%, 50-70%, or >70%) or occluded (Table 2).

Lesions which could not be evaluated were categorized as 0 so that we could exclude a single lesion without excluding the patient.
A CE-MRA or i.a. DSA study was regarded as optimal when all vessel segments could be identified without artifacts due to motion, superimposing of veins (CE-MRA), or inaccurate bolus timing (CE-MRA).
Statistical analysis
The value of agreement between the results of CE-MRA and i.a. DSA and the significance of the study were calculated by using the Wilcoxon matched-pairs test.
The value of sensitivity and specificity as well as the values of positive and negative prediction were calculated on the basis of all stenoses, all occlusions, single vessel segments and especially for stenoses with more than 50% lumen narrowing. To present the rate of correct findings concerning CE-MRA results in correlation with i.a. DSA results, value of efficiency was used. Corresponding interobserver agreement was assessed using Kappa statistics. Interobserver agreement was scored as poor (k <0.1), slight (k = 0.1 to 0.4), fair (k = 0.41 to 0.6), moderate (k = 0.61 to 0.8), or excellent (k = 0.81 to 1.0). For all statistical analysis SPSS for Windows (Version 12.0; SPSS Inc. Chicago, IL, USA) was used.
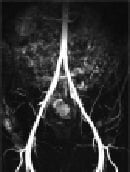
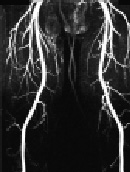
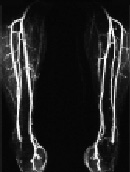
Figure 1. 52-year old male with Claudicatio intermittence stage Fontaine 2a with a walking capacity of 300m.
The MIP images of the CE-MRA of the pelvis (Fig. 1a), the thigh (Fig. 1b), and the calf (Fig. 1c) depicts regular arterial perfusion of both lower extremities without any evidence of stenosis or occlusion. Further invasive diagnostics could be omitted.
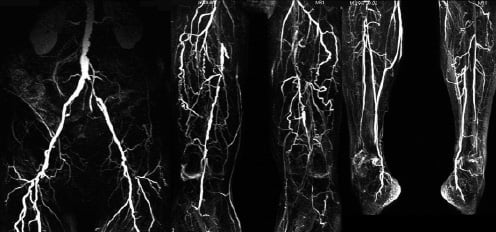
Figure 2. 63 year old male with PAOD stage Fontaine III on both sides.
Fig. 2a: CE-MRA of the pelvic, thigh, and calf region depicts multiple long occlusions on both sides involving the both SFA as well as both popliteal arteries in segment 3. CE-MRA shows still patent vessels at the lower calf (posterior tibial artery on both sides and fibular artery on the right side).
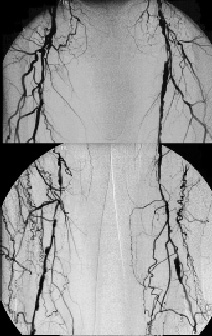
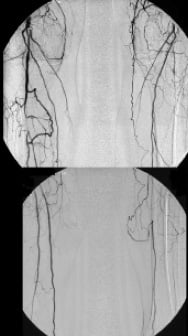
Fig. 2b: i.a. DSA confirms the findings of CE-MRA although the left fibular artery is missing in this examination.
Results
2200 vessel segments from 100 patients (mean age 64.41 ± 10.96), 61 men (mean age 63.01 ± 10.94) and 39 women (mean age 66.90 ± 10.69) with PAOD classified as Fontaine stage 2a (n = 9), 2b (n = 74), 3 (n = 4) or 4 (n = 13) were evaluated employing both CE-MRA and i.a. DSA.
Observers 1 and 2 found vessel morphology in both modalities sufficient for diagnosis in 2063 cases (94.2%) and in 2073 cases (94.7%), respectively (Table 3).
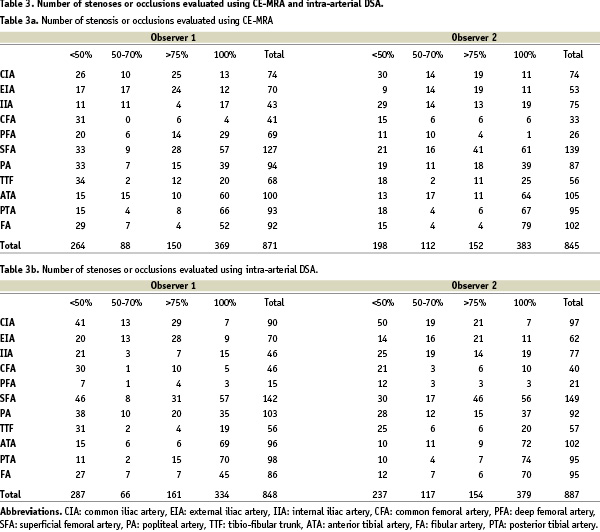
145 stenoses (Observer 2: 161) in the pelvic arteries, 202 stenoses (Observer 2: 187) in the femoropopliteal vessels and 155 stenoses (Observer 2: 123) in the crural arteries were evaluated with CE-MRA. 91 stenoses (Observer 2: 93) in the FOV I, 85 (Observer 2: 112) in the FOV II and 62 (Observer 2: 59) in the FOV III showed a stenosis grade of more than 50%.
We found 42 occlusions (Observer 2: 41) in the pelvic arteries, 129 (Observer 2: 107) within the thigh vessels and 198 occlusions (Observer 2: 235) in the arteries of the lower leg.
There was no significant overestimation in the evaluation of observer 1 concerning the pelvic stenosis (Observer 2: 4/202 [1.98%] Fig. 1). But we found a degree of overestimation of 47/331 (14.2%) (Observer 2: 15/285 [5.3%]) in the results of the femoral stenosis and of 40/353 (11.3%) (Observer 2: 39/358 [10. 9%]) in the stenosis of the lower leg arteries.
Referring to all 871 (Observer 2: 845) stenoses the degree of overestimation after CE-MRA compared to the results of i.a. DSA was 9.98% (87/871) (Observer 2: 6.9% [58/845]). Interventional treatment was suggested of in 72 lesions (Observer 2: 41). The remaining 15 lesions (Observer 2: 17) were found to be more suited for surgical therapy (Fig. 2). In 11 patients additional treatment was suggested by CE-MRA, but could not be confirmed in i.a. DSA. 6 of these patients presented severe stenosis in the region of the calf in CE-MRA. In selective i.a. DSA however, these stenosis were <30%. Two patients presented with severe stenosis in the popliteal artery segment 2, and 3 respectively but only revealed plaque with a stenosis of <10% in i.a. DSA. The remaining 3 patients showed 80% stenosis in the distal external iliac artery, and presented severe stenosis of the common femoral artery in i.a DSA.
The degree of overestimation concerning occlusions was found to be 10.3% (38/369) (Observer 2: 5.7% [22/383]). This resulted in a suggested interventional treatment in 21 (Observer 2: 13) lesions, while in 17 (Observer 2: 9) lesions surgical therapy was found to be indicated (Fig. 3).
The overall sensitivity was calculated at 100% (Observer 2: 95.3%) with an overall specificity of 98.3% (Observer 2: 100%; Table 4).
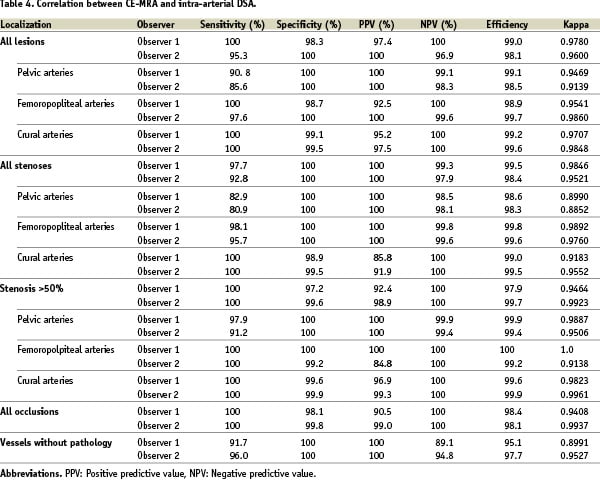
The positive predictive value (Table 4) varied between 100% (Observer 2: 100%) for the pelvic area, 92.2% (Observer 2: 100%) for the femoral arteries, and 95.2% (Observer 2: 97.5%) for the vessels of the lower leg.
The negative predictive value (Table 4) for the iliac vessels was 99.1% (Observer 2: 98.0%), 100% (Observer 2: 99.6%) for the femoral tract, and 100% (Observer 2: 100%) below the trifurcation.
In 784 of 871 (90.0%) stenoses (Observer 2: 787/845 (93.1%) the findings of both examinations were identical.
The value of agreement between the results of CE-MRA and i.a. DSA and the significance of the study were calculated by using the Wilcoxon matched-pairs test (Table 5).
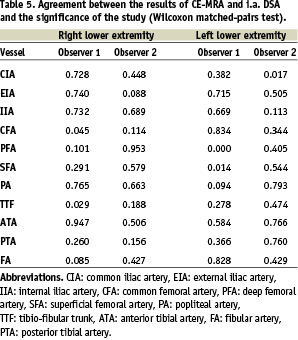
Altogether the value of significance varied between 0.382 and 0.740 (Observer 2: 0.017 and 0.689) for the iliac arteries, 0.000 and 0.834 (Observer 2: 0.114 and 0.953) for the femoropopliteal vessels and 0.029 and 0.947 (Observer 2: 0.188 and 0.766) for the crural arteries.
During the analysis 168 (7.6%) (Observer 2: 161 (7.3%)) of the lesions were excluded. 41 (Observer 2: 44) of them could not be evaluated with the i.a. DSA. 25/41 (60.97%) cases (Observer 2: 30/44 [68.2%]) were unavailable for evaluation due to technical failure and in 16/41 (39.0%) (Observer 2: 14/44 [31.3%]) cases lesions which could not be classified in this study, for example aneurysm and bypass surgery, were detected.
127 lesions (5.8%) (Observer 2: 117 (5.3%) had to be excluded due to the results of CE-MRA. In 12/127 vessel segments (9.5%) (Observer 2: 8/117 [6.8%]) artefacts due to implanted stents occurred. The evaluation of the remaining 105/127 (90.6%) (Observer 2: 109/117 [93.3%]) segments was impossible due to severe motion artefacts, aneurysms, bypass grafts, and technical failures such as shadows of the coil and bad contrast.
Discussion
I.a. DSA is the gold standard of diagnostic imaging technology in peripheral vascular disease. However, recent promising developments in MRA technique and innovations during the last 10 years, such as the volume adjusted contrast enhanced aortography by Prince et al. 199414, three dimensional contrast enhanced MRA by Shetty et al. 199515, MRA with automatic table movement and single bolusing by Ho et al.16 and Voshenrich et al. 199817 and the introduction of the array surface coil10,18 to improve spatial resolution, give reason to compare the validity of MRA and i.a. DSA. Their status in diagnosis and planning of peripheral interventions was evaluated by comparing stenosis grades, occlusions and therapy proposals in 100 symptomatic patients made on the basis of both imaging techniques.
Advantages of MRA, as already mentioned in previous studies, are risk reduction in regard to bleeding and perforation due to the non-invasive nature of the method, the lack of radiation exposure and less time involved. Opposed to this it should be stated that the inability to depict vessel calcification in MRA is a draw back of CE-MRA. Based on the cost-benefit analysis of Meindl et al.19 the additional costs for the MRA can be compensated for with lower staff costs and higher patient comfort.
Joarder et al. has reported20 that MRA allows visualisation of up to 10% more vessels than DSA, especially in the infrapopliteal area. Busch and Hoffmann et al.21 as well as the Berlin multicenter study by Hentsch et al.10 and others18,22 have demonstrated the superiority of MRA when visualizing distal arteries after the occurrence of long occlusions. This fact is supported by the findings by Carpenter et al.23 in 1994 who reported a significant limitation of i.a. DSA in visualizing arteries of the lower leg. He found that 24% of the vessels which became apparent during surgery had not been seen employing preoperative i.a. DSA.
The corresponding value of interobserver agreement 0.978 (Observer 2: 0.960) in our present study confirms the high quality of MRA in comparison to i.a. DSA in the infrapopliteal arteries and disagrees with the opinion of Herborn et al.24 in that the quality of MRA images in peripheral areas cannot be guaranteed without additional measures such as suprapopliteal compression.
Overlapping venous vessels and low spatial resolution in MRA imaging impart a significant limitation to evaluating the size and location of lesions. In the pelvic area an additional difficulty is the superimposition of vessels and bowl. In the peripheral region contrast agents remain significantly longer within the arteries dependant on venous insufficiency than within the iliac vessels. The continuous in- and out-flow of small veins and the difficulty in calculating the resulting effect of dilution and saturation leads to alteration of the signal which cannot be eliminated by established subtraction techniques. A possible solution to this problem can be the time adjusted application of contrast agent or the employment of the mid thigh compression method described by Herborn et al.24.
An improvement in spatial resolution can be achieved by using an array surface coil.
Artefacts caused by stents led to exclusion of 12 (Observer 2: 8) vessels from this study. Also motion artefacts, technical failure due to early or late timing of the imaging and malpositioning of the imaging slices are familiar causes for the exclusion of patients or lesions from evaluation. The latter was not encountered in this study.
In 4 cases the simultaneous bolus adjusted acquisition of both lower extremities with different hemodynamically significant lesions on each side resulted in unequally high signals in the vessels. In some cases this might lead to a false positive evaluation because the diminished or even missing signal on one side could be misinterpreted as a severe stenosis or occlusion.
Another limitation of MRA imaging is the missing display of anatomic structures such as bones which make it difficult to exactly define the transition of the vessels (for example the transition of EIA and CFA or SFA and PA) and led to mismatch in the evaluation of 2 cases.
Additionally, MRA is limited by the relatively high risk of overestimation, which arises either from the 3D images, the maximum intensity projection image reconstruction or from artefacts9,25-27.
An accurate detection of lesions is necessary for the exact depiction of PAOD, but even more important are the therapeutic conclusions drawn from imaging. In our study, the overall sensitivity and specificity of CE-MRA was >95%, and >98% respectively of all investigated segments. Although the rate of disagreement was low, the therapeutic approach based solely on the results of MRA would have led to additional interventions in 11 patients. These cases were either located in the region of the lower limb or the transition of EIA and CFA. Both are locations with known problems for accurate CE-MRA diagnosis. However, CE-MRA in our study showed a positive predictive value of 97%, making additional invasive diagnostic procedures unnecessary in the majority of cases.
The sensitivity and specificity resulting from this study are compatible with corresponding values of previous studies. Ruehm et al.26 reported a value of sensitivity of 92% and of specificity of 96.6% and an interobserver agreement of 0.85 in a study with 61 patients. Winterer et al.28 examined 76 patients and calculated a sensitivity of 100% and specificity of 96% in case of suprainguinal and femoropopliteal vessels and corresponding values of 93% and 96% for the arteries of the lower leg.
Goyen and Debatin29 reviewed 33 articles and reported a mean sensitivity of 93.9% and a mean specificity of 97.1% with a range of 88 to 100% for both values.
Based on the results of this study, drawn from a relatively large number of patients, we conclude that CE-MRA employing a dedicated MRA coil and high dose contrast material is an excellent modality for the screening, treatment planning and follow-up in patients with PAOD and has a diagnostic accuracy comparable to DSA. Especially in patients with restenosis or reocclusion, CE-MRA enables an accurate and non-invasive diagnostic alternative and facilitates treatment planning.

