Abstract
Objective: Both the sirolimus-(SES) and paclitaxel-eluting (PES) stents have been shown to reduce restenosis rates when used in relatively simple lesions. This study aimed to evaluate the results of a consecutive series of patients treated with drug-eluting stent implantation for de novo bifurcation lesions, and compared outcomes with respect to stenting strategy and stent type.
Patients: From April 2002 to September 2003, all patients at our institution were treated with drug-eluting stent implantation. A consecutive series of 144 patients were treated for 167 de novo bifurcation lesions with SES, followed by 104 patients treated with PES for 113 lesions.
Results: Clinical follow-up at 6 months was obtained in 99% patients with survival-free of major adverse cardiac events (MACE) of 93.7% for SES versus 85.8% for PES, p=0.05. By multivariate analysis, factors predictive for MACE were age, diabetes mellitus, previous CABG, multivessel disease, treatment for acute myocardial infarction, and treatment with PES. Survival-free of target lesion revascularization (TLR) was 95.7% for SES versus 86.8% for PES, p=0.01, with stent type being the only independent predictor. Technique of stenting was not a predictor of either MACE or TLR.
Conclusions: MACE rates for both the SES and PES are low compared with historical data of bare metal stents. The most effective techniques for bifurcation stenting remain undefined. Our data suggests a higher need for TLR for the PES compared with the SES, however further randomized studies are needed to fully evaluate both stenting strategy, and any difference between the stents.
Introduction
The outcome of percutaneous therapy (PCI) of bifurcation lesions with bare metal stents is hindered by an increased rate of procedural complications1, and a high rate of restenosis particularly when both the main vessel and side branch are stented2,3,4,5,6. The advent of drug-eluting stents is revolutionising the practice of interventional cardiology by demonstrating a reduction in the subsequent rate of restenosis. There is evidence of efficacy in randomized trials for both the sirolimus- (SES) and paclitaxel-eluting (PES) stents for the treatment of relatively simple lesions7,8. In addition, the sirolimus-eluting stent for the treatment of bifurcation lesions has demonstrated a low rate of adverse cardiac events compared with historical data utilizing bare metal stents9,10. However, the most effective technique of stenting for bifurcation lesions with drug-eluting stents is currently unknown. In the present report we evaluate the rate of major adverse cardiac events following PCI for bifurcation lesions treated with either SESs or PESs in a consecutive series of patients. In addition, outcomes were assessed with respect to the baseline bifurcation anatomy and type of stenting strategy employed.
Methods
Bifurcation classification: All lesions were classified on baseline angiography according to the Duke classification (figure 1).
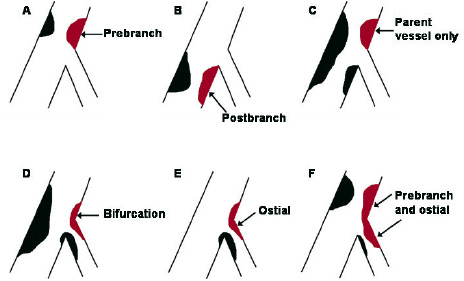
Fig. 1: The Duke classification of bifurcation lesions.
Procedure: The sirolimus-eluting stent (Cypher™, Johnson & Johnson - Cordis unit) received CE mark approval in April 2002. Since that time, all patients undergoing percutaneous therapy in our institution have been treated with drug-eluting stent implantation as the default strategy. During the first quarter of 2003, our strategy switched from the sirolimus- to the paclitaxel-eluting stent (Boston Scientific) enabling a comparison of the two stent types. All consecutive patients were enrolled irrespective of clinical presentation and lesion characteristics, and the incidence of major adverse cardiac events (MACE) was prospectively evaluated during the follow-up.
All procedures were performed with standard interventional techniques. The strategy of bifurcation stenting employed, and the use of kissing balloon dilatation post-procedure were at the operators’ discretion. One of 6 methods of stenting was used: stenting of the main vessel with balloon-only angioplasty of the side branch; type A T-stenting (stenting first of the side branch, followed by stenting of the main vessel); type B T-stenting (stenting of the main vessel followed by stenting of the side branch because of a sub-optimal result)2; the ‘crush’ technique11; culotte stenting12; or kissing stents (simultaneous implantation in the main vessel and side branch with the proximal edges of the stents side by side). SESs were available in diameters from 2.25 mm to 3.00 mm and lengths from 8 mm to 33 mm. PESs were available in diameters from 2.25 mm to 3.5 mm and lengths from 8mm to 32mm. During the procedure, intravenous heparin was given to maintain an activated clotting time ≥250 seconds. Patients were preloaded with 300 mg clopidogrel, and received life-long aspirin together with 75 mg clopidogrel per day for 6-months. The use of glycoprotein IIb/IIIa inhibitors was at the discretion of the operator. The protocol was approved by the Institutional ethics committee and is in accordance with the principles of Good Clinical Practice for Trials of Medicinal Products in the European Community and the Declaration of Helsinki. All patients signed a written informed consent
Follow-up: Clinical follow-up was obtained using telephone calls and questionnaires, and evaluated the rate of major adverse cardiac events (MACE) which were pre-defined as death, acute myocardial infarction (AMI), or target vessel revascularization (TVR). The diagnosis of AMI required an elevation of creatine kinase levels to twice the upper limit of normal, together with a rise in creatine kinase-MB fraction. Target lesion revascularization was defined as either surgical or percutaneous reintervention driven by significant (>50%) luminal diameter narrowing either within the stent or the 5mm borders proximal and distal to the stent, and was undertaken in the presence of either anginal symptoms or objective evidence of ischemia. Target vessel revascularization was defined as revascularization within the target vessel including encompassing the target lesion. The definition of stent thrombosis was the presence of intra-stent thrombosis, with or without stent occlusion, documented on angiography, and was categorized as acute if occurring within 24 hours or subacute if within 30 days after stent implantation.
Statistical analysis: Discrete variables are presented as percentages and compared with Fisher exact test. Continuous variables are expressed as mean ± standard deviation and compared with Student’s t test. Cumulative survival and MACE-free survival were calculated according to the Kaplan-Meier method. The log-rank test was used to compare MACE-free survival between the two groups. All tests were two-tailed, and a p value of <0.05 was considered as significant. Logistic regression models were established to investigate independent predictors of MACE (death, AMI, or TVR), and target lesion revascularization. Variables entered were age, gender, diabetes mellitus, hypertension, hypercholesterolemia, smoking, multivessel disease, prior AMI, prior CABG, clinical presentation, use of a glycoprotein IIb/IIIa inhibitor, target vessel, bifurcation anatomy, stent type, stenting technique, diameter of stent, total length of stents, and use of kissing balloon post-dilatation. Odds ratio with corresponding 95% confidence intervals are reported.
Results
The baseline patient and procedural characteristics for the SES and PES cohorts are presented in tables 1 and 2 respectively. There were no significant differences between the 2 groups with respect to baseline patient characteristics, though there was a trend towards an increased usage of glycoprotein IIb/IIIa inhibitors in the PES group (38.5% versus 27.8% in the SES group, p=0.07). There was no significant difference in the number of stents used, however, the mean nominal diameter of stent used in the main vessel was greater with the PES (2.93 ± 0.34mm versus 2.85 ± 0.23 for the SES, p=0.007). For those patients treated with stent implantation in the side branch, though there was no significant difference in the number of stents used, the total length of stented segment in the side branch was longer for the PES-treated patients (18.8 ± 10.5mm versus 14.1 ± 7.6mm, p=0.0001). The choice of stenting strategy during the 2 treatment periods is presented in figure 2. The total number of lesions treated with each stenting technique was single stent utilization in 55 (19.6%), type A T-stenting in 47 (16.8%), type B T-stenting in 46 (16.4%), crush stenting in 88 (31.4%), culotte stenting in 24 (8.6%), and kissing stents in 20 (7.1%). There was no difference with respect to the use of kissing balloon post-dilatation between the SES and PES cohorts.
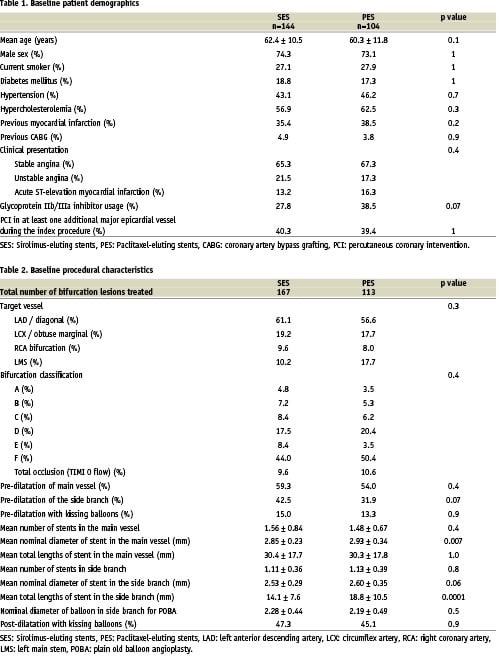
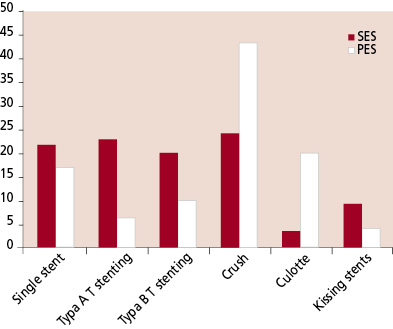
Fig. 2: The type of stenting strategy employed for the Sirolimus- eluting (SES) and Paclitaxel-eluting stent (PES).
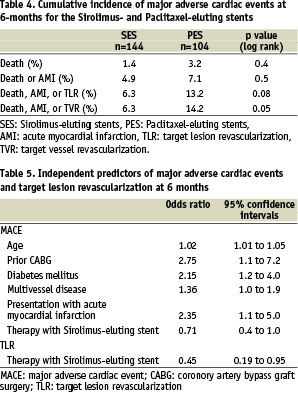
Clinical follow-up was obtained in 99.2% patients. Angiographically documented stent thrombosis occurred in 2 patients treated with SES (1.4%) and 3 patients treated with PES (2.9%), p=0.4 (Table 3). All episodes of stent thrombosis were subacute (within 30 days following stent implantation), and were treated percutaneously, all patients survived. The cumulative incidence of major adverse cardiac events at 6-months for the SES and PES groups are presented in Table 4, and the survival-free of MACE at 6-months is illustrated in figure 3. The independent predictors for MACE and TLR by multivariate analysis are shown in Table 5. The only factor found to be predictive for TLR was stent type. Neither the baseline bifurcation anatomy, nor the type of stenting strategy utilized, were predictive of events.
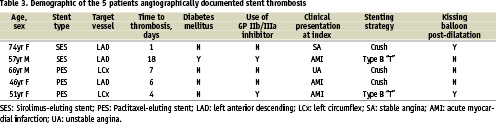
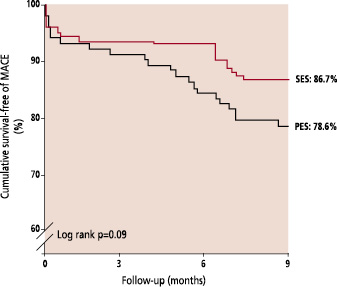
Fig. 3: Kaplan-Meier curves for survival-free of major adverse cardiac events (MACE) for the Sirolimus-eluting (SES) and Paclitaxel-eluting stent (PES).
At 6-months, survival-free of TLR was 95.7% for SES versus 86.8% for PES, p=0.01 (figure 4). TLR was for subacute thrombosis in 5 patients (see above), was for restenosis of the main vessel in 4 lesions treated with SES (2.4%) and 6 lesions treated with PES (5.3%), for restenosis of the side branch in 3 lesions treated with SES (1.8%) and 3 treated with PES (2.7%), and for restenosis of both branches in 2 lesions treated with SES (1.2%) and 2 treated with PES (1.8%).
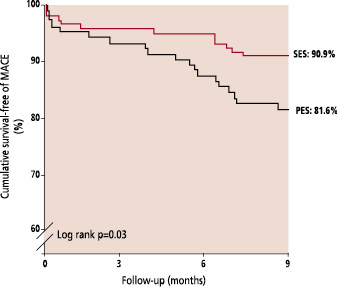
Fig. 4: Kaplan-Meier curves for survival-free of target lesion revascularization (TLR) for the Sirolimus-eluting (SES) and Paclitaxel-eluting stent (PES).
Discussion
In the present report we have demonstrated low rates of major adverse cardiac events at 6-months for both the sirolimus- and paclitaxel-eluting stents when used for the treatment of de novo bifurcation lesions. Independent predictors for MACE were age, diabetes mellitus, multivessel disease, previous CABG, treatment in the setting of acute myocardial infarction, and therapy with PES. Target lesion revascularization (TLR) at 6-months was higher in the PES group than the SES group, with a survival-free of TLR of 86.8% versus 95.7% respectively, p=0.01. By multivariate analysis, the use of PES was the only factor predictive for TLR.
The most effective strategy for the treatment of bifurcation lesions with drug-eluting stents is currently unknown. In the present study, the choice of stenting strategy was at the operators’ discretion. Previous data from our group following bifurcation stenting with the SES, demonstrated an overall restenosis rate of 23%9. The majority of restenoses of the side branch occurred at the ostium following T-stenting. Indeed, the restenosis rate in the side branch following T-stenting was 16.7% whilst that following other stenting techniques was 7.1%. We hypothesised that these restenoses might relate to inadequate / incomplete coverage of the ostium of the side branch thereby reducing the efficacy of the drug-eluting stent. This led to a shift away from a strategy of T-stenting, towards methods which ensure complete coverage - the crush and culotte techniques of stenting (figure 2). One potential disadvantage of these strategies however, is that they lead to an area of double or triple layer of stent struts raising theoretical concerns that the increased dosage of drug at this site might induce endothelial dysfunction and potentiate the risk of thrombosis. Despite the change in stenting technique in the present study, the choice of strategy was not an independent predictor for either MACE or the need for TLR. The current study is limited by the lack of angiographic follow up, so cannot fully evaluate restenosis which, particularly when occurring in the side branch, may be clinically silent.
Currently, there is only one published randomized evaluation of drug-eluting stents for bifurcation lesions10. This randomized 85 patients to a single SES with balloon-angioplasty of the side branch, versus implantation of 2 SESs. The overall rate of restenosis at 6 months was 26% (19% in the single stent group versus 28% in the double stent group, p=NS). However, the study was limited by the high crossover rate with 51% of the patients in the single stent group crossing to the double stent group because of a suboptimal result in the side branch. In addition, the approach to stenting technique was not uniform. However, both this randomized study, and the registry data from our group demonstrate an improvement in the restenosis rates compared with historical data of bare metal stenting.
Restenosis following bare stent implantation is related to the length of stent, and inversely related to the diameter13. The majority of TLRs were for restenosis within the main vessel stent, yet the nominal stent diameter was actually bigger for the PES. This probably related to a larger available diameter of PES (3.5mm versus 3.0mm for the SES), and throughout the study, post-dilatation was carried out whenever necessary. The mean total length of stent used in the side branch of the PES group was significantly longer than the SES group. However, neither stent diameter nor length was an independent predictor for subsequent MACE or need for TLR.
Previous data of bare metal stent implantation in bifurcation lesions, demonstrate rates of target lesion revascularization of between 16% and 38%2,3,4,5,6. Compared with this historical data, in the current study, TLR was certainly lower for the SES (survival-free of TLR of 95.7% at 6 months). However, multivariate analysis demonstrated a significantly higher need for TLR following stenting with the PES compared with the SES, with the majority of TLRs in the main vessel. This might reflect a difference in the efficacy of the 2 drugs, at least at the current dosages, or relate to differences in stent design14. The SES is a closed-design stent whereby each cell is bound on all sides with the junction of each strut pair joined to another strut pair junction. The PES however, is an open-cell design meaning that some of the junction nodes are unattached within the stent structure. A previous of 54 patients undergoing elective stenting showed that platelet activation was lower in those receiving a closed versus open-cell designed stent15. In the present study, though not significantly different between the 2 groups, subacute thrombosis did occur in a higher percentage of the PES patients (2.9% versus 1.4%, p=0.4). The same authors15 examined stent implantation in the pig model and found that more tissue prolapse occurred following implantation of a stent with an open cell design. Both the SES and PES have been evaluated in large randomized studies and compared with their respective bare stents (Bx Velocity™ and Express™)16,17. Though the inclusion criteria in these studies were not absolutely identical, both studies were very similar and included patients with stable or unstable angina and single de novo lesions; bifurcation lesions were excluded. Both the mean lesion length, and reference vessel diameter were similar. Evaluation of the angiographic follow-up of those treated with bare stents, showed a mean in-stent lumen loss of 1.00 ± 0.70mm in SIRIUS (Bx Velocity™), and 0.92 ± 0.58mm in TAXUS-IV (Express™). The higher late lumen loss in the Bx Velocity™ stent conflicts with the suggestion that the lower TLR rate with SES in the present study might relate to the difference in stent design. Both the SES and PES are covered by polymer coatings to facilitate drug-elution. Previous evaluation of other polymers has suggested that these can in themselves promote varying degrees of an inflammatory response and restenosis18. In the same randomized studies, evaluation of the drug-eluting stent cohorts showed a mean in-stent late loss of 0.17 ± 0.45mm in SIRIUS, and 0.39 ± 0.50mm in TAXUS-IV, perhaps suggesting the SES is more efficacious at inhibiting the development of neointimal hyperplasia than the PES.
Interpretation of the results of the present study with respect to stent type is limited by the lack of randomization. The REALITY study is a multicenter evaluation of more than 1300 patients with multivessel disease, randomized to either SES or PES implantation. Initial results were recently presented at the American College of Cardiology meeting in 200519. There was no significant difference with respect to the overall rates of MACE between the stent types (9.2% for SES versus 10.6% for PES, p=0.41). However, in keeping with the difference in the degree of platelet activation related to stent design15, the rate of stent thrombosis was higher for the PES group (1.8% versus 0.4%, p=0.0196). Furthermore, all angiographic parameters with respect to efficacy of suppression of neointimal growth were better following SES implantation. The in-stent late loss was 0.09 ± 0.43mm for the SES, versus 0.31 ± 0.44mm for the PES, p<0.001. Such a difference may potentially be clinically relevant when treating complex lesions such as bifurcations, particularly when vessels with a small diameter are stented. Patients with bifurcation lesions were not excluded from this study, and a more detailed analysis of subgroups such as those treated for a bifurcation lesion is awaited.
The most effective strategy for percutaneous therapy of bifurcation lesions with drug-eluting stents needs to be carefully evaluated in future studies. Interpretation of future randomized studies should take into account baseline anatomical differences of bifurcation lesions as the best strategy for a true bifurcation lesion (involving both the main vessel and side branch) may not necessarily be the same as that for lesions affecting only one of the branches. In addition, restenosis particularly at the side branch may not always lead to a recurrence in symptoms and follow-up angiography should be carried out to fully evaluate the results.
Study limitations
The major limitations of this study are that it is a single centre registry and is non-randomized, with the choice of stenting strategy left entirely at the operators’ discretion. In addition, routine angiographic follow-up data was not obtained, and additional restenoses giving rise to minimal / no symptoms, particularly at the ostium of the side branch, cannot be excluded. However, clinical follow-up data was available for >99% providing an accurate reflection of the rate of clinically important adverse events following therapy of bifurcation lesions in a consecutive series of patients without exclusion.
Conclusions
The use of both the sirolimus- and paclitaxel-eluting stents for the treatment of de novo bifurcation lesions appears feasible and safe, both demonstrating low rates of major adverse cardiac events at 6-months. The increased rate of target lesion revascularization following PES implantation needs to be further evaluated in a randomized fashion, and at present, the most appropriate technique for bifurcation stenting with drug-eluting stents remains unclear.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1.
Moving image 2.
Moving image 3.
Moving image 4.
Moving image 5.
Moving image 6.

