Abstract
Transcatheter aortic valve implantation (TAVI) has become an established treatment option for symptomatic patients suffering from aortic stenosis. A bicuspid aortic valve (BAV) is the most frequent congenital valvular abnormality. With the expansion of the indications for TAVI to patients at lower risk, including younger populations, a BAV is expected to be more frequently encountered. Several challenges are associated with BAVs, from diagnosis and classification to interventional or surgical treatment. Transcatheter prostheses, designed to treat tricuspid aortic valves, have shown promising results in BAV anatomies. However, technical limitations, such as underexpansion, ellipticity or procedural complications, have been identified. Several issues for transcatheter procedures are still a matter of discussion. In this state-of-the-art review, we explore the knowledge acquired about TAVI for BAVs, the sizing and technical specificities of interventional procedures, as well as the remaining evidence gaps and future perspectives.
Abicuspid aortic valve (BAV) is the most common congenital structural heart abnormality, affecting 0.5% to 2% of the general population and found in 5% to 20% of transcatheter aortic valve implantation (TAVI) populations in the Western world1. The epidemiology of BAV may be different in Asia, as approximately 50% of patients presenting for TAVI in China are reported to have a BAV morphology2. However, this may simply reflect the younger age of these populations, as the prevalence of a BAV is much higher in younger aortic stenosis (AS) patients compared to older patients. BAV anatomy predisposes to early valve degeneration, which may lead to severe AS and/or aortic regurgitation (AR) and a need for surgical aortic valve replacement (SAVR) at a young age3. Surgery has long been the gold-standard treatment for severe symptomatic bicuspid AS and remains the first-choice treatment for most young BAV patients with low surgical risk. For older BAV patients and those at high surgical risk, TAVI has become an accepted and appropriate treatment option. Contemporary TAVI in selected bicuspid AS patients has been reported to have similar clinical outcomes compared to TAVI in tricuspid AS patients45. However, due to relevant anatomical differences, BAVs may be associated with an increased risk of suboptimal procedural outcomes and complications in case of TAVI (Central illustration), underscoring the need for careful patient selection and the use of patient-tailored TAVI strategies to achieve optimal outcomes6. This review article aims to give a state-of-the-art overview of our knowledge on TAVI in bicuspid AS and to discuss future directions.
Classification
Classification of BAV disease is essential for understanding its clinical presentation and guiding therapeutic strategies, particularly in the context of TAVI. Over the years, several classification systems have been proposed to categorise BAV anatomy based on cusp morphology, the presence of a raphe, and commissural orientation (Figure 1). The Sievers classification, based on the number of raphes, remains the most broadly adopted in daily clinical practice, although it does not cover the full complexity of BAV anatomy7. It divides BAVs into three phenotypes: type 0, when there is no raphe; type 1, when there is a raphe connecting two cusps; and type 2, when two raphes are identified. Sievers type 1 BAV, with a raphe between the right and left coronary cusps, is the most common phenotype in Western TAVI populations. Type 0 BAV seems to be more frequently encountered in Asian TAVI populations, while type 2 BAV is a rare phenotype worldwide and could more accurately be termed a unicuspid instead of a bicuspid aortic valve17. With the advent of multislice computed tomography (MSCT) imaging for TAVI procedural planning, Jilaihawi et al proposed another classification based on computed tomography (CT)-derived anatomical insights, categorising BAVs into bi- and tricommissural types8. Although it addresses some of the limitations of the Sievers classification and better evaluates supra-annular structures for TAVI planning, its adoption in daily clinical practice remains infrequent. The International Consensus Statement, introduced in 2021, offers a more comprehensive and clinically relevant definition of a BAV, complementing the Sievers and Jilaihawi classifications9. It classifies BAV anatomy into three major phenotypes: two-sinus, fused, and partial-fusion types. The system further incorporates cusp symmetry, commissural angles, and the presence and characteristics of a raphe. For example, the fused BAV type, which is the most common, involves the fusion of two cusps – most frequently the right and left coronary cusps – with a raphe that may or may not be calcified. The International Consensus Statement enables a precise BAV classification, facilitating procedural TAVI planning. This classification also identifies a partial-fusion BAV and excludes unicuspid variants, unlike the Sievers classification. However, the concept of a fused BAV may be somewhat misleading because “developmentally” this phenotype is the result of incomplete cusp separation and must be distinguished from a fused commissure in tricuspid aortic valves.
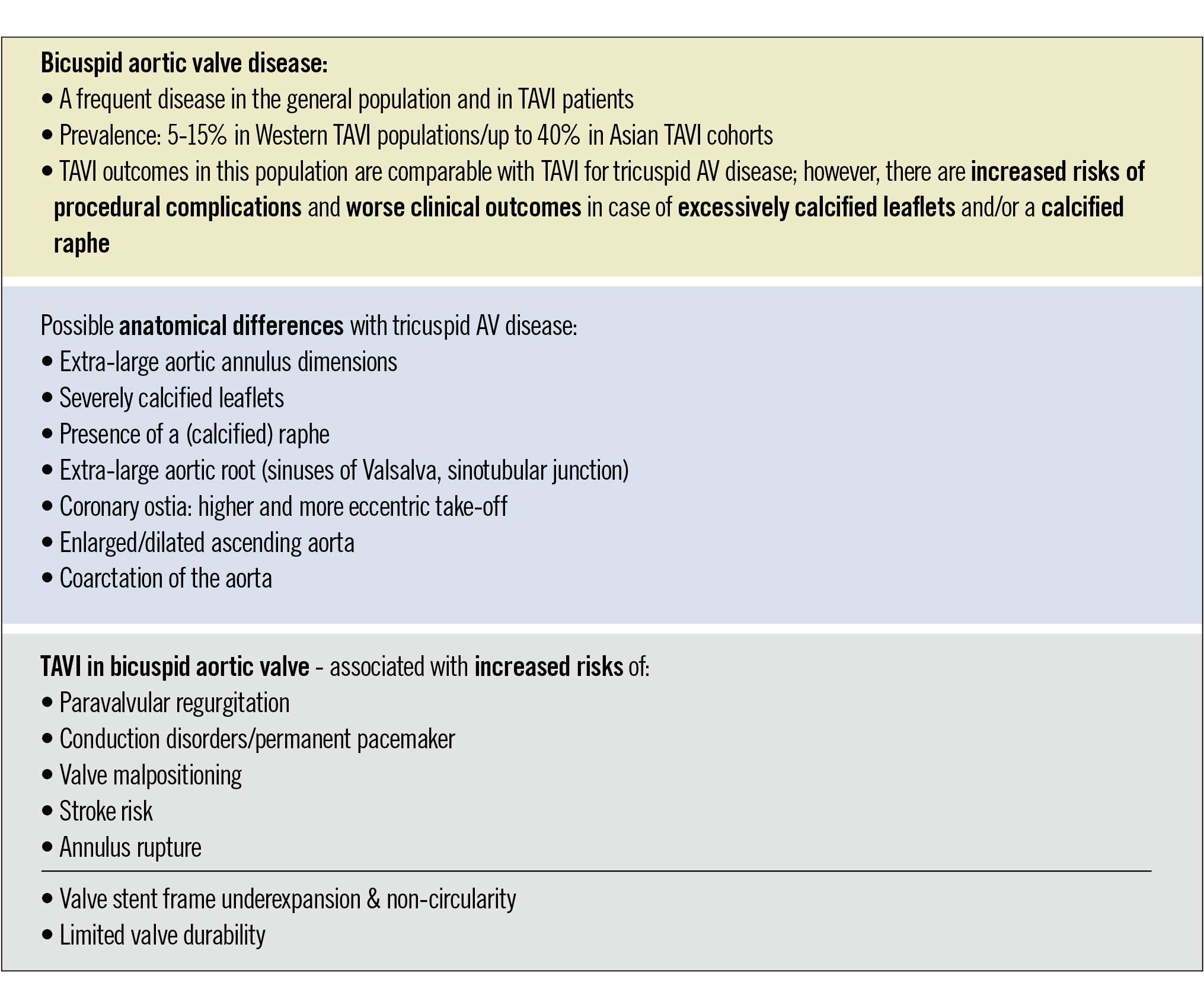
Central illustration. Key features of TAVI in patients with bicuspid aortic valve disease, including anatomical differences with tricuspid aortic valve disease, and specific associated risks. AV: aortic valve; TAVI: transcatheter aortic valve implantation
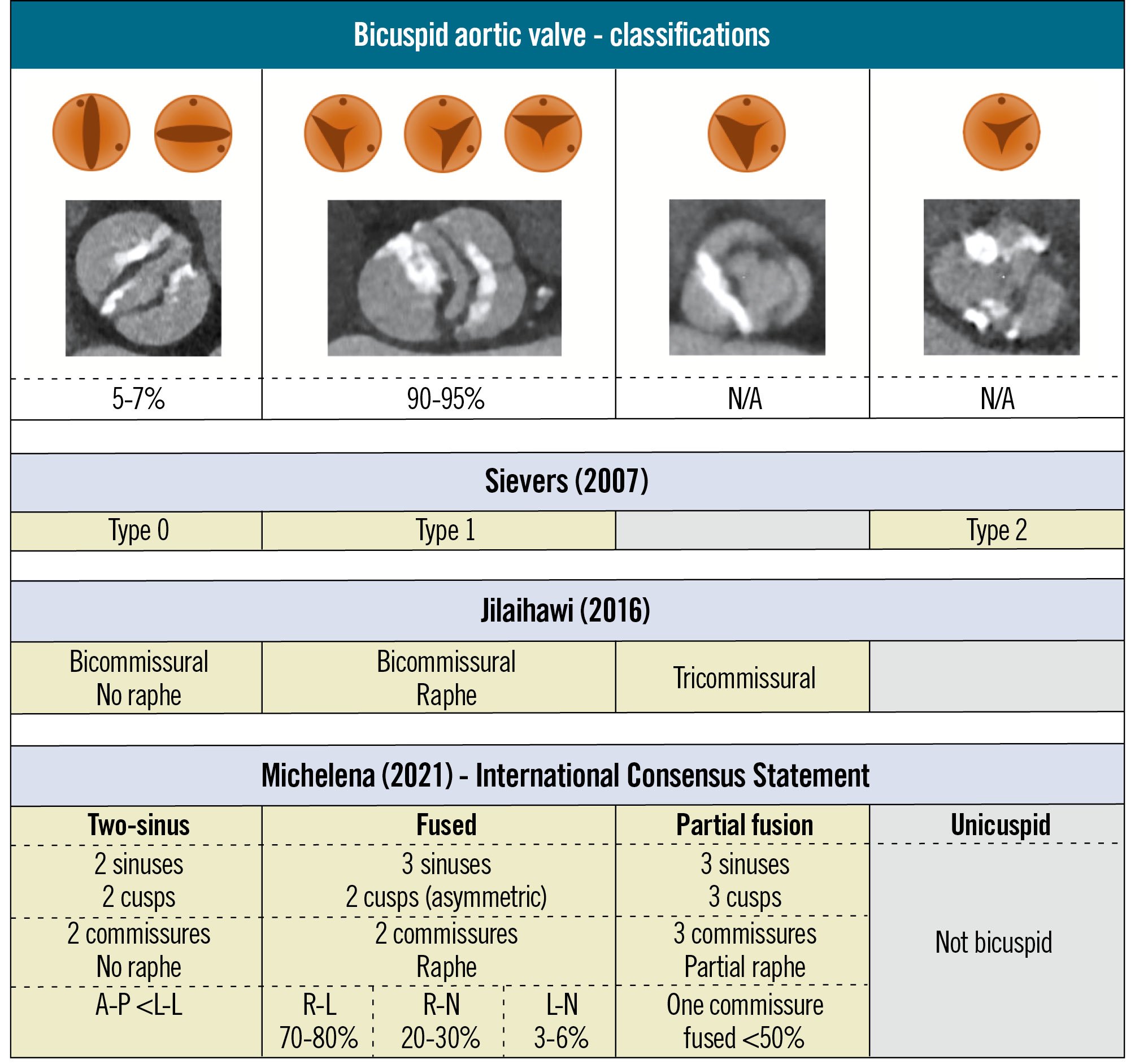
Figure 1. Bicuspid aortic valve classifications. Different classification systems to categorise bicuspid aortic valve anatomy based on cusp morphology, the number of commissures, and the presence of a raphe. A-P: anterior-posterior; L-L: latero-lateral; L-N: left-non-cusp; N/A: not available; R-L: right-left cusp; R-N: right-non-cusp
Surgical treatment of BAV disease
SAVR remains the gold-standard treatment for patients with severe symptomatic BAV disease, in particular for patients less than 75 years of age and with no prohibitive surgical risk. Surgical intervention is also indicated for asymptomatic patients who have severe AS (aortic valve area <0.8 cm²) or significant AR, especially if (1) there is evidence of left ventricular impairment (left ventricular ejection fraction <50% or significant ventricular dilation) or (2) the patient exhibits a rapid progression of their valve disease during follow-up. Both mechanical and bioprosthetic valves are options, depending on the patient’s age, preferences, and anticoagulation considerations. Combined procedures are decided based on the association with any aortopathy, concomitant mitral disease, coronary artery disease, or rhythm disturbance10. Individuals with a BAV are at a higher risk for aortopathy, including conditions such as aortic dilation and aneurysm. This risk is particularly pronounced in patients with associated connective tissue disorders, such as Marfan syndrome or Loeys-Dietz syndrome, in which structural abnormalities of the aortic wall exist11. The reported prevalence of aortic dilatation in BAV patients is approximately 50%, and the proposed mechanisms for aortic dilation are haemodynamic and/or genetic12. Both the European and US guidelines consider that concomitant aortic surgery is a reasonable approach (Class IIa) in BAV patients undergoing surgery for severe AS or AR with a dilated aortic root or ascending aorta of ≥45 mm, as this may prevent future complications such as aortic dissection or rupture. The latest ESC/EACTS guidelines broaden the indication for TAVI to include BAV patients who are at high surgical risk and have favourable anatomy, after thorough assessment at a Heart Valve Centre1314.
Recent data on TAVI in BAV disease
Registry data
Technological advancements in devices, combined with a more comprehensive understanding of BAV anatomy, have enabled the application of transcatheter therapies to treat patients with BAV disease. Excluded from major randomised controlled trials (RCTs), patients with BAV stenosis have been predominantly studied in retrospective registries and only a few prospective studies. Table 1 summarises the findings of the most important studies. One of the first and largest multicentre retrospective studies was published in 201415. Mylotte et al analysed the early and medium-term safety and efficacy outcomes of TAVI in a cohort of 139 patients with a BAV, using either self-expanding valves (SEVs) or balloon-expandable valves (BEVs). Most patients (63%) presented with a bicuspid Sievers type 1 morphology. At 30 days, the mortality rate was 5%, and the device success rate reached 90%, with no significant differences observed between the two prosthesis groups. At 1-year follow-up, Kaplan-Meier analysis indicated a mortality rate of 17.5%, with congestive heart failure as the primary cause of death. This initial registry demonstrated TAVI feasibility in BAV disease and highlighted the role of CT analysis as a potential factor for improving transcatheter aortic valve (TAV) prosthesis type and size selection and, consequently, patient outcomes. Another multicentre registry exclusively utilising the balloon-expandable SAPIEN 3 TAV (Edwards Lifesciences) in a population of bicuspid AS patients demonstrated favourable valve performance and a minimal rate of paravalvular regurgitation (PVR) at 30-day follow-up16. The study enrolled a total of 51 patients (82% Sievers type 1). At the 30-day follow-up, no cases of moderate or severe PVR were reported, with two deaths (3.9%) and an overall device success rate of 98%. The authors underscored the low rate of PVR as one of the key findings of the study, attributing it to the technological features of the prosthesis, including the external sealing skirt on the inflow portion, as well as the improved valve delivery system, which enhanced accuracy during positioning. While the study demonstrated promising early results, these should be interpreted with caution given its retrospective design and the small sample size. A comparable outcome with the SAPIEN 3 valve was reported by Attinger-Toller et al in a multicentre study conducted some years later, with a median follow-up of 390 days17. Next, a retrospective propensity-matched analysis of 561 bicuspid and 4,546 tricuspid AS patients showed a higher frequency of conversion to surgery, a lower device success rate, and a higher incidence of moderate or greater PVR in the bicuspid cohort18. This study demonstrated that the outcomes were influenced by the generation of TAV being used: BAV patients treated with early-generation devices experienced more frequent aortic root injuries with BEVs and a higher incidence of moderate-to-severe PVR with SEVs. Another multicentre registry explored the association and impact of BAV anatomical features on TAVI outcomes in 1,034 bicuspid AS patients19. The presence of a calcified raphe and excessive leaflet calcium was associated with worse clinical outcomes (higher 2-year all-cause mortality compared to patients with one or neither of these features [25.7% vs 9.5% vs 5.9%; p<0.001]). The role of BAV anatomical features was further highlighted in the AD HOC registry20. This study included 946 patients with bicuspid AS Sievers type 1. Independent predictors of PVR included a large virtual raphe ring perimeter, severe annular or left ventricular outflow tract (LVOT) calcification, use of a SEV, and intentional supra-annular TAV positioning. Although this study offered valuable insights, some important limitations should also be recognised, such as its retrospective study design, the inclusion of a variety of TAV devices, and the lack of a standardised sizing method.
Table 1. Main registries and trials exploring TAVI for bicuspid aortic valves.
| Year, first author | Study design, sample size | Primary endpoint | Main conclusion(s) |
|---|---|---|---|
| 2014, Mylotte D15 | Registry N=139 | Procedural and clinical (VARC) | TAVI in bicuspid AS is achievable with promising short- and medium-term clinical outcomes |
| 2016, Perlman G16 | Registry N=51 | Procedural and clinical (VARC-2) | Promising early results with a device success rate of 98% |
| 2017, Yoon SH18 | Registry (PS-matched analysis) N=561 | Procedural and clinical (VARC-2) | In comparison to TAVI in tricuspid AS: similar clinical outcomes, but a lower device success rate in the bicuspid cohort |
| 2019, Waksman R23 | Registry N=61 | All-cause mortality at 30 days | No mortality at 30 days |
| 2019, Attinger-Toller A17 | Registry N=79 | Procedural and clinical (VARC-2) | Favourable outcomes at 1 year and beyond, with a device success rate of 98% |
| 2020, Yoon SH19 | Registry N=1,034 | All-cause mortality at 1 and 2 years | High-risk morphological features in BA influence outcome |
| 2021, Makkar RR24 | Registry (PS-matched analyses) N=37,660 (3,243 bicuspid and 34,417 tricuspid) | 30-day and 1-year mortality and stroke | No significant difference between the groups |
| 2021, Forrest JK26 | Evolut Low Risk bicuspid study registry N=150 | Incidence of all-cause mortality or disabling stroke at 30 days | Favourable 30-day outcome |
| 2022, PARTNER 3 bicuspid registry, Williams MR25 | Registry (PS-matched analyses) N=169 BA* vs 496 TA | 1-year composite rate of death, stroke, and cardiovascular rehospitalisation | No significant difference between the groups |
| 2022, Majmundar M28 | Registry (PS-matched analyses) N=17,068 patients with a BAV (1,629 TAVI and 15,439 SAVR) | In-hospital mortality | TAVI is associated with reduced rates of in-hospital mortality |
| 2023, BIVOLUTX, Tchétché D21 | Registry N=149 | Valve performance at 30 days | Favourable valve performance at 30 days |
| 2024, Evolut Low Risk Bicuspid Study, 3-year follow-up, Zahr F27 | Registry N=data available for 128 patients | Rates of all-cause mortality or disabling stroke | Low rates of all-cause mortality or disabling stroke |
| 2024, AD HOC, Zito A20 | Registry N=946 | PVR incidence, MAE | Moderate or severe PVR occurred in about 4%; PVR ≥moderate was linked to an increased risk of MAE |
| 2024, NOTION-2, Jørgensen TH5 |
Randomised trial (TAVI vs SAVR) N=370 randomised, 100 patients with a BA | Composite of all-cause mortality, stroke, or rehospitalisation at 12 months |
In the BA cohort, the rate of the composite endpoint was significantly higher in patients undergoing TAVI |
| *Highly selected bicuspid anatomy; patients were excluded if presenting one of the following: left ventricular outflow tract or raphe calcification, aortic annulus diameter <16 mm or >28 mm, and ascending aorta diameter >40 mm. AS: aortic stenosis; BA: bicuspid anatomy; BAV: bicuspid aortic valve; BE: balloon-expandable; MAE: major adverse events (all-cause death, stroke, or hospitalisation for heart failure); PS: propensity score; PVR: paravalvular regurgitation; SAVR: surgical aortic valve replacement; SE: self-expanding; TA: tricuspid anatomy; TAVI: transcatheter aortic valve implantation; VARC: Valve Academic Research Consortium | |||
Impact of sizing technique
The Bicuspid Aortic Stenosis With Evolut Platform International Experience (BIVOLUTX) study was a multicentre registry that aimed to assess the performance of the self-expanding, supra-annular Evolut PRO/XL valve (Medtronic) in 149 bicuspid AS patients (mean Society of Thoracic Surgeons Predicted Risk of Mortality [STS-PROM] score 2.6%) undergoing TAVI21. The CT analysis included various sizing methods, all of which were reviewed by a dedicated core lab. At 30 days, the cardiac death rate was 2.6%, with the valve demonstrating optimal haemodynamic performance. Moderate PVR was reported in 3 patients (2.8%), with no cases of severe regurgitation. At 1-year follow-up, valve haemodynamics remained consistent. Only 1 patient experienced moderate-to-severe PVR, and 3 patients had severe patient-prosthesis mismatch. Different CT-based sizing methods did not impact device or clinical outcomes. The BIVOLUTX study has limitations: 86.5% of all patients had a BAV of Sievers type 1. Consequently, the study findings should not be generalised to other BAV phenotypes, and the limited sample size (for other BAV phenotypes) did not allow for comparisons between BAV phenotypes.
Low-risk patients
TAVI for low-risk patients was approved by the U.S. Food and Drug Administration (FDA) in 201922. The Low Risk TAVR (LRT) study was the first to evaluate low-risk patients with bicuspid AS undergoing TAVI with either BEVs or SEVs23. At 30 days, there were no reported cases of mortality or ischaemic stroke. One patient experienced moderate PVR. Excellent clinical short-term results were reported in this low-risk population. Another large propensity-matched study, including 3,168 pairs of low surgical risk bicuspid and tricuspid AS patients (mean age: 69 years) treated with the balloon-expandable SAPIEN 3 valve showed no significant difference in the primary endpoint of death and stroke at 30 days and 1 year24. Similar findings were observed in the PARTNER 3 bicuspid registry25. This study included 169 low surgical risk bicuspid AS patients (primarily Sievers type 1) with a mean age of 71.0 years. A propensity score-matched analysis was also conducted comparing tricuspid AS patients treated in the Evolut Low Risk trial and bicuspid AS patients from the Evolut Low Risk bicuspid registry. No significant differences were observed between groups26. The 3-year follow-up data were recently published27. At 3-year follow-up, similar outcomes were observed in both groups regarding all-cause mortality or disabling stroke. This study represents the longest follow-up to date for low surgical risk bicuspid AS patients, offering insights into the valve durability of this particular TAV prosthesis in this population.
TAVI versus SAVR
Adjunctive data on outcomes for TAVI and SAVR in bicuspid AS were derived from a propensity score analysis of a cohort of 17,068 patients from the Nationwide Readmission Database (NRD), which generated 1,393 matched pairs28. The results showed that TAVI was associated with lower in-hospital mortality, with similar rates of major adverse cardiovascular events at 1 and 6 months. The favourable outcomes observed after TAVI in bicuspid AS should be interpreted with caution. This is because critical information was missing, such as valve anatomy and TAV prosthesis selection. The Nordic Aortic Valve Intervention Trial 2 (NOTION-2) randomised 370 patients with severe AS and a mean age of 71.1 years at low surgical risk (median STS-PROM score 1.1%) to either TAVI or SAVR5. Among this cohort, 100 patients had a BAV, primarily Sievers type 1. At 1 year, the incidence of the primary endpoint − a composite of all-cause mortality, stroke, or rehospitalisation − was higher in bicuspid AS patients who underwent TAVI: 14.3% compared to 3.9% for SAVR (hazard ratio 3.8, 95% confidence interval: 0.8-18.5). The study included different BAV anatomical morphologies, complexities, and prostheses. This underscores the need for a properly sized randomised trial comparing TAVI and SAVR in bicuspid AS patients.
Sizing methods for TAVI in BAVs
Similar to TAVI in tricuspid AS, the gold standard for procedural planning of TAVI in BAVs consists of an in-depth analysis of MSCT images of the aortic valve (AV) complex and aortic root at different levels both above and below the virtual aortic annulus (Figure 2). The CT “scroll technique” offers a comprehensive overview of the anatomy during the mid-systolic phase of the cardiac cycle. This technique involves scrolling through the MSCT images from the level of the LVOT up to the ascending aorta to assess key anatomical features, such as BAV cusp morphology, calcium extent and distribution, raphe appearance and location, and coronary ostia location. In BAVs, these features can present very different challenges, each of which may impact TAV choice and procedural outcomes. Furthermore, it is important to evaluate the dimensions of the ascending aorta and aortic arch, as a BAV can be associated with dilatation of the ascending aorta and coarctation of the aorta (Figure 2). The choice of a correct TAV size is critical for safeguarding the procedural success of TAVI in BAVs while also minimising complications. The specific anatomical features of a BAV – including asymmetrical and excessive leaflet calcification and a (calcified) raphe – require tailored sizing methods in order to guarantee procedural success and reduce the risks of procedural complications. Several sizing methodologies have been proposed, each with its advantages and limitations (Figure 3). 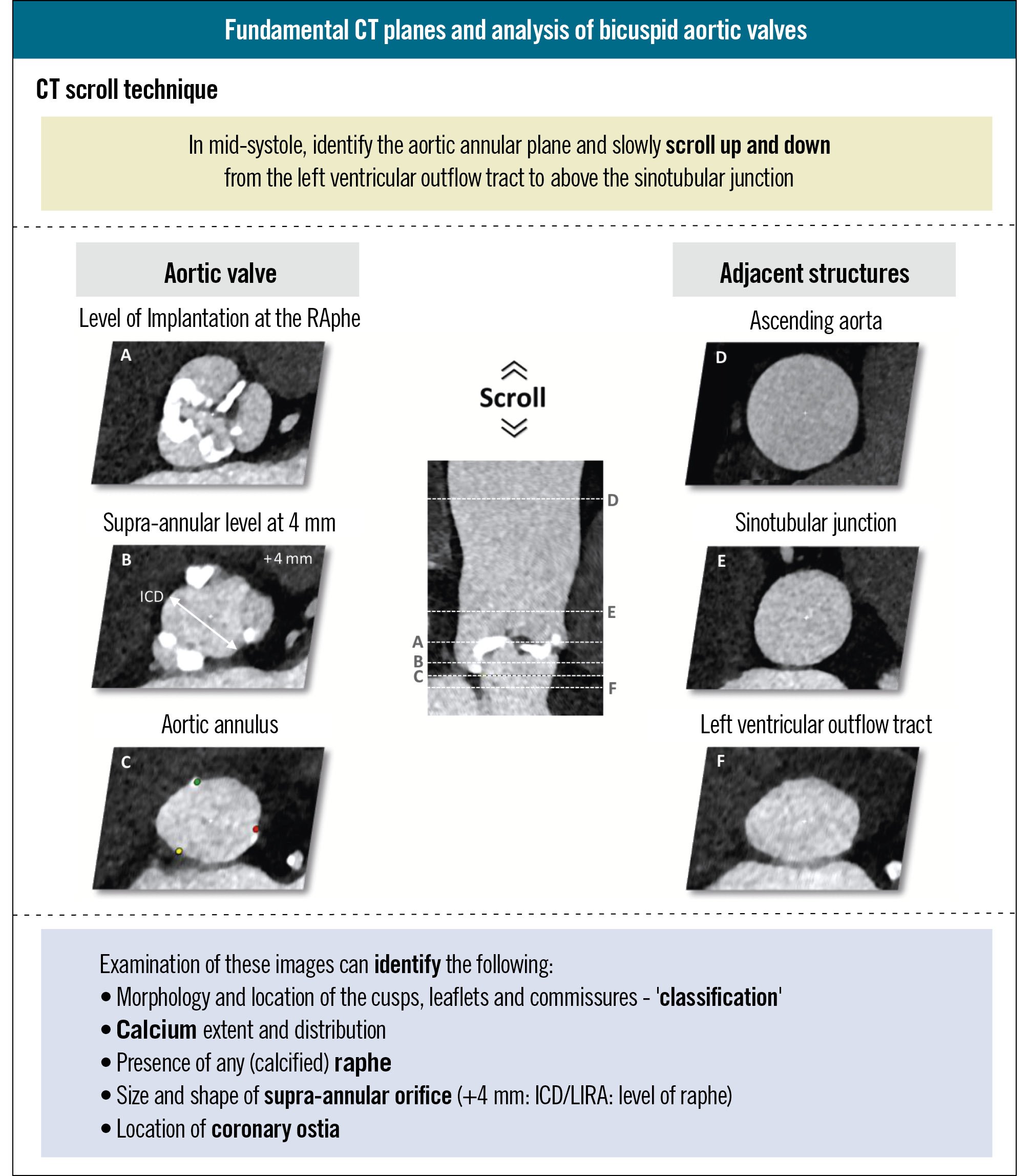
Figure 2. Fundamentals of CT analysis of bicuspid aortic valves for TAVI treatment planning. The CT scroll technique ensures a comprehensive assessment of the aortic valve complex and aortic root. Precise measurements are made at different planes, including some specific planes to bicuspid aortic valve disease. CT: computed tomography; ICD: intercommissural distance; LIRA: Level of Implantation at the RAphe; TAVI: transcatheter aortic valve implantation
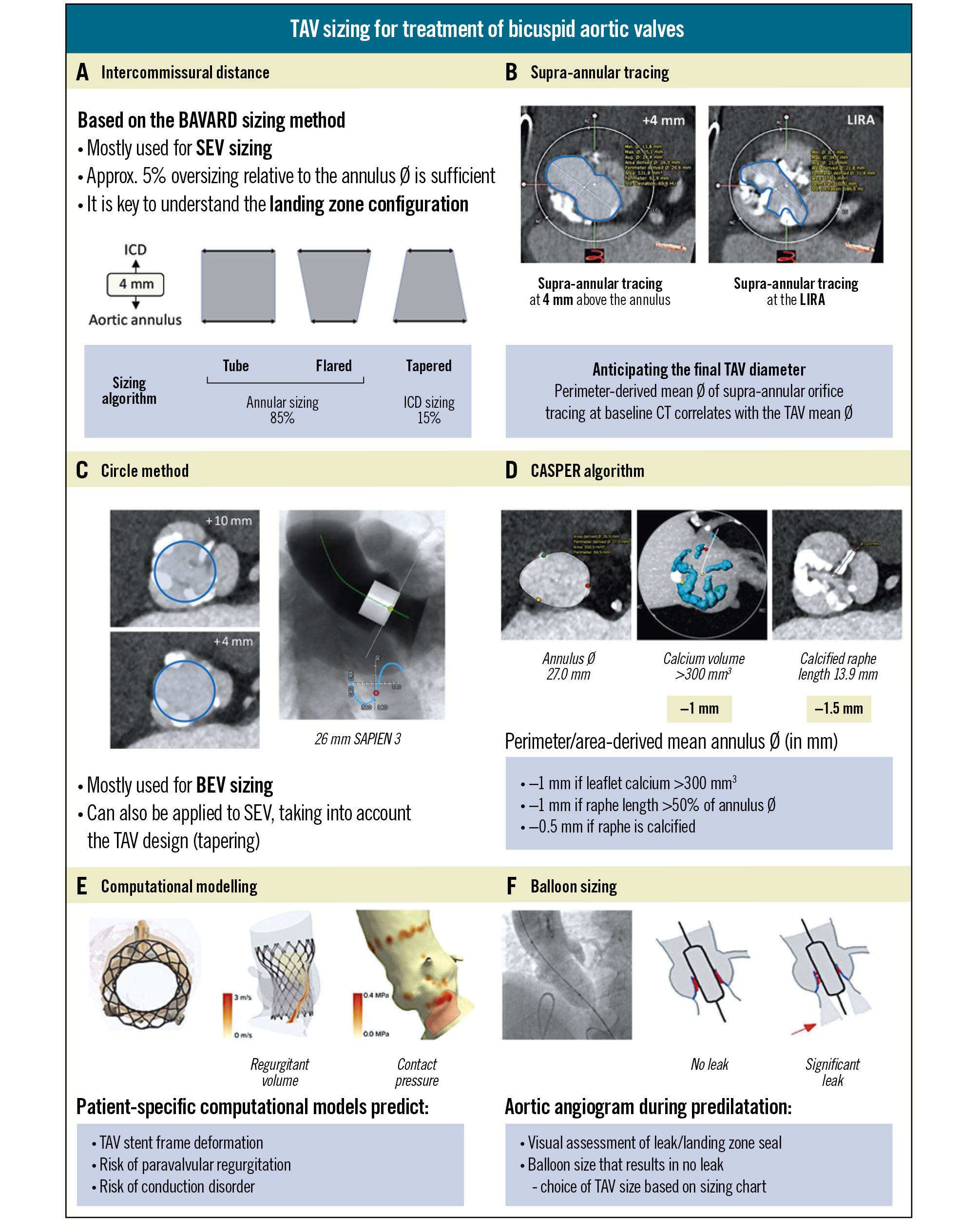
Figure 3. Different TAV sizing methodologies for the treatment of bicuspid aortic valves. A) Intercommissural distance, based on the BAVARD sizing method. B) Supra-annular tracing. C) Circle method. D) CASPER algorithm. E) Computational modelling. F) Balloon sizing. BAVARD: Bicuspid Aortic Valve Anatomy and Relationship with Devices; BEV: balloon-expandable valve; CASPER: Calcium Algorithm Sizing for bicusPid Evaluation with Raphe; CT: computed tomography; ICD: intercommissural distance; LIRA: Level of Implantation at the RAphe; SEV: self-expanding valve; TAV: transcatheter aortic valve
Intercommissural distance
The Bicuspid Aortic Valve Anatomy and Relationship with Devices (BAVARD) study was a multicentre registry study aimed at evaluating the anatomical characteristics of BAVs and their impact on TAVI outcomes4. When treating tricuspid AS with TAVI, the aortic annulus dimensions are considered for TAV size selection. However, the investigators realised that it is crucial to understand the configuration of the entire landing zone when treating bicuspid AS with TAVI. In an effort to achieve standardisation and according to the BAVARD sizing algorithm, measurement of the intercommissural distance (ICD) at 4 mm above the aortic annulus was introduced. Based on the comparison of the mean aortic annulus diameter and the ICD at 4 mm above the annulus, the BAV landing zone can be categorised as a tube, flared, or tapered. In case of a tube or flared configuration (85% of cases), standard TAV sizing based on the annular dimensions is recommended, whereas TAV “downsizing” is recommended in case of a tapered configuration of the landing zone (15% of cases) (Figure 3A). This BAVARD sizing method has been validated in the BIVOLUTX study using the Evolut PRO/XL TAV and has been adopted in several high-volume centres in daily clinical practice thanks to its ease of use and reproducibility21. Of note, this sizing method is primarily validated for and applicable to TAVI with SEVs.
Supra-annular tracing
Another approach to get a better insight and understanding of the BAV landing zone has been tracing the supra-annular orifice. In theory, these tracings can be performed at any level above the aortic annulus. However, sizing techniques for TAVI in BAVs relying on tracings of the supra-annular orifice have been described at specific, predefined levels above the virtual aortic annulus42029. In parallel to the ICD measurement, the BAVARD investigators propose tracing the supra-annular orifice at 4 mm above the aortic annulus. The rationale behind this method is that the perimeter-derived mean diameter of the supra-annular tracing correlates strongly with the final mean TAV diameter and, as such, can assist in the final TAV size selection. On the other hand, the Level of Implantation at the RAphe (LIRA) sizing method relies on tracing the supra-annular orifice at the level of maximal raphe protrusion (approximately 10 mm above the annulus)3031. However, the LIRA method is not widely adopted due to poor reproducibility and its applicability being limited to BAVs with a long, calcified raphe (Figure 3B).
Circle method
The “circle method” involves drawing concentric circles at the annulus and every 3 mm above the annulus on MSCT images to assess which valve size would best fit the anatomy. Therefore, it is of utmost importance to assess whether a chosen TAV size covers the commissures (at 4 mm above the annulus) and fits well within the supra-annular orifice at the level of the calcified leaflets and/or (calcified) raphe (Figure 3C). Hence, the circle method integrates the BAVARD and supra-annular tracing methods into one comprehensive approach. Clearly, this sizing method offers simplicity and ease of use, allowing a straightforward visual assessment of the “valve fit” into a given anatomy. On the other hand, its performance depends heavily on operator experience, and it has only been validated for TAVI with BEVs32. However, when using dedicated CT analysis software such as 3mensio (Pie Medical Imaging), a “virtual valve” with the exact dimensions of the chosen valve type and size can be superimposed onto the CT images and can offer this same visual assessment of “valve fit” into the anatomy for any TAV.
CASPER algorithm
The Calcium Algorithm Sizing for bicusPid Evaluation with Raphe (CASPER) algorithm uses a formula starting with the aortic annular dimension and then subtracting 0.5-1 mm based on factors such as calcium burden and raphe length to determine TAV size (Figure 3D)33. A strength of this sizing method is that it offers a standardised approach with high reproducibility. On the other hand, an important limitation of this method is that it has only been validated in small sample sizes using early-generation devices (including a mechanically expandable TAV, which is no longer available).
Computational modelling
Computational modelling is a newer technology which relies on artificial intelligence-based simulations to predict TAV stent frame deformation, the risk of PVR, and potential conduction disorders for a specific TAV type and size in a patient-specific anatomy (Figure 3E)3435. This highly individualised approach allows for meticulous procedural planning, including simulations with different TAV designs and sizes. While computational modelling has shown value when planning for TAVI in complex bicuspid cases, its reliance on specialised software and service providers has limited its accessibility in routine clinical practice. Computational modelling also shows promise with regard to the planning of redo-TAVI in younger bicuspid patients, with valuable insights into the risk of coronary occlusion and coronary inaccessibility in redo-TAVI scenarios.
Balloon sizing
The balloon-sizing method involves inflation of a balloon in the native aortic valve (predilatation) while performing an aorta angiogram to assess the presence of contrast leak into the left ventricle (Figure 3F). In case of doubt between two TAV sizes, it is recommended to perform balloon sizing with a balloon diameter matching the smaller TAV size (for BEV) or one at the lower end of the aortic annulus range recommended for the smaller TAV size (for SEV). If no leak is detected, the balloon size is considered adequate, and the corresponding TAV size can be selected. While this method is straightforward and offers a real-time assessment of the valve’s landing zone seal, a drawback of this sizing method is that it is performed intraprocedurally, thereby delaying TAV selection and loading. Additionally, the absence of contrast leak does not always guarantee optimal seal with the final valve implantation.
Procedural considerations
When performing TAVI in BAV patients, there are several considerations to address the specific anatomical complexities of BAVs (Figure 4). A tailored approach is essential to maximise procedural success and reduce the risks of procedural complications, such as annulus rupture, sinus perforation, valve migration, PVR, and TAV underexpansion9. At each step in the procedure, patient-tailored modifications can be pivotal for achieving optimal outcomes. Of note, cerebral embolic protection may be considered when performing TAVI in BAV patients given the increased risk of procedural stroke in these patients536. 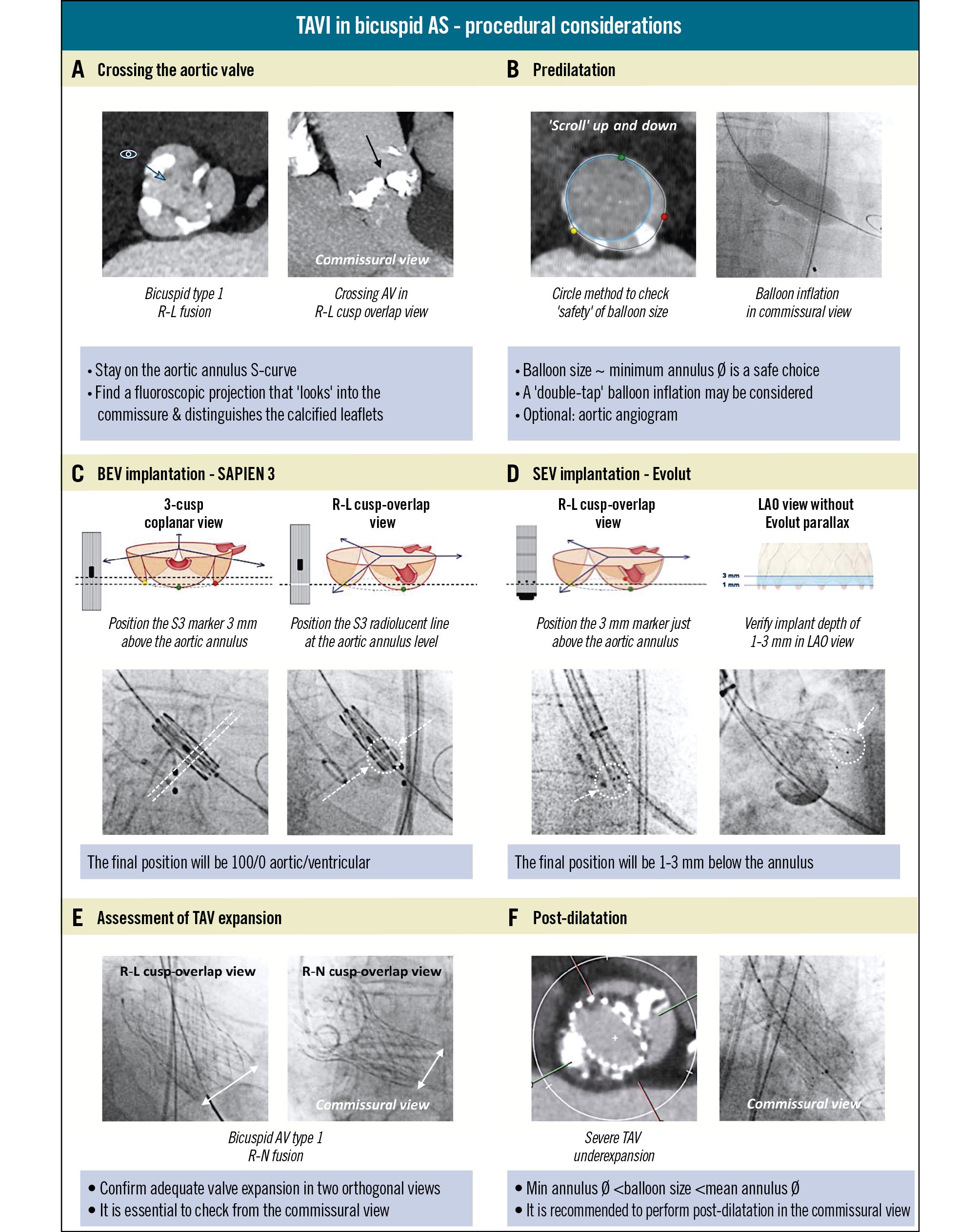
Figure 4. Procedural considerations for TAVI in bicuspid aortic stenosis. A) Tailoring fluoroscopic projections for crossing the aortic valve. B) Aortic valve balloon predilatation. C, D) Positioning and implantation of a BEV or SEV in a bicuspid aortic valve stenosis. E) Assessment of post-implant TAV expansion. F) TAV balloon post-dilatation. AS: aortic stenosis; AV: aortic valve; BEV: balloon-expandable valve; LAO: left anterior oblique; R-L: right-left cusp; R-N: right-non-cusp; S3: SAPIEN 3; SEV: self-expanding valve; TAV: transcatheter aortic valve; TAVI: transcatheter aortic valve implantation
Crossing the aortic valve
Wire crossing of BAVs can be difficult because of extensive leaflet calcification, a (calcified) raphe, and the presence of a wide and/or horizontal aortic root. Use of a predetermined patient-tailored fluoroscopic projection – the commissural view – derived from the preprocedural cardiac CT, can facilitate this step and is crucial throughout the entire procedure. The commissural view is a fluoroscopic projection on the aortic annulus S-curve, which is in line with the commissure, looking into it and distinguishing the calcified leaflets (Figure 4A)37. In case of the most common BAV phenotype − a BAV Sievers type 1 with right-left fused cusps − the commissural view corresponds to the classical right-left cusp-overlap view.
Predilatation
Predilatation is commonly performed when treating BAVs with TAVI, and it serves two primary purposes: facilitating the crossing of the native aortic valve with the TAVI delivery system and promoting optimal TAV expansion, thereby reducing the risk of TAV underexpansion (Figure 4B). This step is particularly important for SEV platforms, where TAV underexpansion can lead to TAV infolding, migration, or even embolisation. The balloon size for predilatation should be carefully matched to the patient’s anatomy, taking into account the anatomical size and extent of calcifications at the level of the aortic annulus, LVOT, and aortic root. A safe balloon size should be based on preprocedural CT planning using the circle method or the minimum annulus diameter. During predilatation, use of the commissural view allows for the assessment of balloon expansion and calcified leaflet modification. In case of severely calcified leaflets, a “double-tap” balloon inflation may be considered in order to maximally modify the leaflet calcifications. An aortic angiogram can also be performed during predilatation to assist with TAV sizing.
TAV implantation
The choice of TAV type – either BEV or SEV – has implications for the implantation strategy when treating BAVs. For both types, higher implants are typically targeted in BAVs, because a deeper implant position increases the risks of supraskirt PVR and conduction disturbances (Figure 4C-Figure 4D)438. For BEVs, this higher TAV implant position corresponds to a 90/10% or even 100/0% supra-annular/infra-annular position, which can be achieved by deploying a SAPIEN valve (Edwards Lifesciences) with the radiopaque marker 3 mm above the annulus in the three-cusp coplanar view and/or with the radiolucent line at the annulus level in the right-left cusp-overlap view39. Moreover, when positioning BEVs in a BAV anatomy, it is important to verify that the outflow part of the BEV is positioned at least 1 mm above the dense leaflet calcifications; this to avoid TAV embolisation towards the left ventricle – this is a particular point of attention when using the shorter-frame Myval BEV (Meril Life Sciences) in bicuspid valves. For SEVs, this higher TAV implant position corresponds to a 1 to 3 mm implant depth in relation to the aortic annulus. In case of implanting the Evolut platform, the right-left cusp-overlap view is typically used to guide initial TAV positioning, thereby keeping the 3 mm radiopaque marker just above the aortic annulus level during deployment to achieve a final depth of 1 to 3 mm below the annulus. Before final valve release, it is important to verify a minimum implant depth of 1 mm below the left coronary cusp; this can be done in a left anterior oblique (LAO) fluoroscopic projection with no parallax in the TAV. Although targeting higher TAV implants improves procedural outcomes, it may compromise redo-TAVI options. In case of redo-TAVI, a high index TAV implant may result in a relatively high leaflet neoskirt, which could increase the risk of coronary artery occlusion (due to sinus sequestration) and coronary inaccessibility40. This is particularly relevant when planning for TAVI in bicuspid AS patients, as these patients are (on average) younger and have a longer life expectancy compared to tricuspid AS patients. In very selected BAV cases with severe (right) coronary ostium eccentricity, it has been suggested by some operators to use the coronary ostia overlap fluoroscopic projection for SEV implantation; however, this may not be practically feasible and may complicate coronary access to the left coronary artery and jeopardise the benefits of commissural alignment41.
Assessment of TAV expansion
Following implantation, assessing TAV expansion is crucial to ensure that the valve is well positioned and fully expanded. Therefore, it is essential to use two orthogonal fluoroscopic views, one LAO and one right anterior oblique fluoroscopic view, with one of these being the commissural view; this allows for a reliable evaluation of TAV symmetry and expansion (Figure 4E). This is particularly relevant in BAVs with heavily calcified leaflets and/or a calcified raphe, which can make complete stent frame expansion challenging19. Importantly, there is increasing evidence that TAV underexpansion and eccentricity in BAVs can impact valve haemodynamics, the risk of leaflet thickening, and clinical outcomes42. Hence, this necessitates further intervention to optimise TAV performance.
Post-dilatation
Post-dilatation can improve TAV expansion and circularity and enhance sealing, ensuring optimal valve function and reducing PVR, especially when heavy calcification has hindered full TAV expansion419. A balloon size 1 or 2 mm larger than the minimum annulus diameter – but smaller than the mean annulus diameter – can often be considered a safe and effective choice for post-dilatation. Extra caution and a more conservative balloon choice could be warranted for anatomies with subannular/LVOT calcification (consider a high balloon position) or a narrow and calcified sinotubular junction (consider a low balloon position), as studies with BEVs have linked this to an increased risk of aortic root injury43. Post-dilatation is best performed using the bicuspid “commissural view” for fluoroscopic guidance to assess balloon inflation and TAV stent frame expansion (Figure 4F).
Missing evidence and perspectives
Despite the data on TAVI in BAV patients that have accumulated in recent years, there are remaining knowledge gaps to be filled in order to better understand the outcomes associated with TAVI in BAVs.
Indications and patient selection
Although the European Society of Cardiology/European Association for Cardio-Thoracic Surgery (ESC/EACTS) Guidelines for the management of valvular heart disease formulate clear recommendations on indications for intervention in AS and the recommended mode of intervention, there is hardly any discussion on the role of TAVI in patients with BAV disease. The ESC/EACTS Guidelines state that “While several registries have reported excellent outcomes of TAVI in patients with a BAV who were unsuitable for surgery, SAVR remains more appropriate in patients with aortic stenosis affecting a bicuspid valve and in those with associated disease (e.g., aortic root dilatation, complex coronary artery disease, or severe mitral regurgitation) requiring a surgical approach.” In the ACC/AHA Guidelines for the management of patients with valvular heart disease, a separate chapter has been dedicated to bicuspid aortic valves. However, the ACC/AHA Guidelines are also rather scarce with their comments on the role of TAVI to treat bicuspid AS and mention that “considerations are the younger age of patients with a BAV, for whom the risk-benefit ratio of TAVI versus SAVR needs careful consideration. Randomised controlled trials are needed to obtain full clarity on the optimal use of TAVI in this population, as well as long-term outcomes.” Besides this missing RCT evidence to support TAVI in BAV disease, there is an equally large gap in evidence on which BAV morphologies are suitable, less suitable, or absolutely not suitable for treatment with TAVI. Even an expert consensus document on the sizing and positioning of the SAPIEN 3/SAPIEN 3 Ultra (Edwards Lifesciences) in bicuspid AS did not address the topic of which BAV morphologies are most favourable or, conversely, should be avoided for TAVI and preferably referred to SAVR32. Clearly, a more granular risk stratification is necessary to guide our decision-making in daily clinical practice. Dedicated studies focusing on TAVI outcomes in different BAV morphologies or phenotypes (including BAV Sievers type 2 or unicuspid AV) are required in the future. Finally, there is also missing evidence on what the mode of intervention should be in elderly patients with severe symptomatic AS and concomitant aortic root dilatation. A recent study reported that aortic root dilatation remained stable in 85% of patients at a median follow-up of 3 years after TAVI. In the 15% of patients with continuous aortic root dilatation, TAV stent frame geometry and function were identified as predictive factors of this continued dilatation after TAVI44. However, more evidence is needed on this topic.
Dedicated TAV prostheses
Although only the Evolut and SAPIEN platforms are European Conformity (CE) marked with an indication for TAVI in bicuspid AS, most contemporary balloon-expandable or self-expanding TAVs have been used to treat BAV morphologies; however, this has inherent constraints and weaknesses. Newer-generation TAV devices, such as the Evolut FX+ (Medtronic) and SAPIEN 3 Ultra, have shown promise in optimising TAV positioning, promoting coronary access and preventing PVR in tricuspid AS4546. Prospective studies exploring the performance of these newest-generation devices in bicuspid AS patients are also needed. Importantly, TAV stent frame underexpansion and ellipticity in bicuspid AS could adversely impact valve haemodynamics and durability. Dedicated TAVI devices could be needed in the future to overcome the technical limitations of the current TAVs, which are primarily designed for the treatment of tricuspid aortic valves.
Procedural refinements
There may be significant differences in outcomes based on operator experience with TAVI in BAV patients. Training and the establishment of centres of excellence may help address these disparities. In the future, artificial intelligence could help integrate the bicuspid phenotype and calcium burden into the procedural decision algorithm, and robotic-assisted TAVI could improve the accuracy of TAV positioning and implantation. Furthermore, as many procedural challenges and difficulties are related to the bicuspid configuration of the leaflets – resulting in TAV underexpansion and eccentricity – adjunctive therapies such as leaflet modification may play a role in the future. The ShortCut leaflet splitting device (Pi-Cardia) has shown promise for redo-TAVI procedures in degenerated surgical or transcatheter aortic bioprostheses47. Future studies should investigate whether “tricuspidalisation” of a bicuspid valve, by splitting the fused leaflet, is feasible, safe and effective and whether it might become an indispensable procedural refinement when treating bicuspid anatomies with TAVI.
Long-term outcomes
While several registries have reported excellent outcomes of TAVI in patients with a BAV, most existing evidence is derived from short- to medium-term follow-up studies. Taking into consideration that TAV underexpansion and eccentricity have been extensively documented in bicuspid anatomies, concerns have been raised that this could lead to impaired valve function and favour adverse outcomes such as leaflet thrombosis or valve deterioration48. Long-term outcomes with regard to valve haemodynamics, leaflet thrombosis, valve durability, and reintervention following TAVI in BAV patients are still underresearched and underreported. Understanding how different types of TAVs function over time in this specific anatomical setting is crucial. Larger cohorts of patients with extended follow-up, ideally up to 10 years, are needed to ensure that TAV durability is satisfactory when treating BAVs. Lastly, there is a scarcity of data on patient-reported outcomes and on how TAVI impacts quality of life when treating younger, low-risk patients with a BAV, despite this being an important aspect of assessing treatment effectiveness. Aspects such as functional improvement and impact on quality of life should be considered equally important as valve durability in this group of patients.
Need for comparative studies
When critically evaluating the data on TAVI in bicuspid AS (see section “Recent data on TAVI in BAV disease”), it is clear that most of the current evidence comes from (retrospective) single-arm registry studies. One of the obvious limitations of these registry studies is a strong patient selection bias. So far, there has been no RCT directly comparing TAVI with SAVR in BAV patients. Such studies are needed to determine the best treatment strategy for these patients. A future RCT comparing TAVI with SAVR in BAVs should attempt to enrol the broadest possible bicuspid AS population in the RCT arm, rather than in parallel TAVI or SAVR registry studies. However, it must be accepted that some BAV phenotypes, such as BAV Sievers type 2 or unicuspid aortic valves, will be excluded from the comparative study arm due to the unpredictable TAVI outcomes in these particular bicuspid AV phenotypes. Finally, TAV type and design may also influence procedural and long-term outcomes after TAVI in BAVs. Various commercially available TAVI devices may perform differently in the context of BAVs. Head-to-head comparative studies, comparing TAVI devices in BAV patients are also needed to evaluate the safety and efficacy of different TAV designs in patients with bicuspid anatomy.
Conclusions
TAVI for bicuspid aortic valves has become a mature procedure with excellent clinical outcomes achieved in selected patients. We reviewed the classifications, challenges associated with bicuspid anatomies, and current knowledge and techniques for TAVI in this patient population, from the sizing to the procedure itself. Several remaining issues need to be addressed in future properly sized studies and randomised controlled trials.
Conflict of interest statement
D. Tchétché received consulting fees from Abbott, Boston Scientific, Edwards Lifesciences, Medtronic, and Meril Life Sciences; and has minor shares in Pi-Cardia. V. Cesario received a fellowship training grant from EAPCI, sponsored by Edwards Lifesciences. O. De Backer received institutional research grants and consulting fees from Abbott, Boston Scientific, and Medtronic. Y. Willemen has no conflicts of interest to declare.

