Abstract
Bone marrow-derived cells are involved in the cardiac repair/regeneration. In order to reduce the ischaemic injury the BM cells must undergo mobilisation into peripheral blood and subsequent homing and engraftment into the target organ. The pool of mobilised cells contains committed lineages, but also small population of cells with the potential for tissue repair - endothelial progenitor cells, hematopoietic progenitors, mesenchymal cells and other populations of putative multipotent tissue-committed progenitors. Stem cells mobilisation is mediated by the chemokine/chemokine receptor axes SDF-1/CXCR4, VEGF/VEGFR, HGF/c-met and CD117/SCF. Several factors can influence the mobilisation of EPC from the bone marrow as well as the number and functional capacity of BM progenitor cells in particular advanced age, presence of diabetes and use of statins are the most important factors associated with the number and function of mobilised cells. The recently published data from the prospective analysis of patients with stable coronary artery disease showed that mobilisation of stem/progenitor cells may have prognostic value. The number of circulating CD34+ cells is correlated with left ventricular ejection fraction and remodelling and the number of progenitor cells may be the predictor of the improvement in global and regional left ventricular contractility after the myocardial infarction.
Background
Bone marrow-derived cells are involved in the cardiac repair/regeneration as shown in various animal models of the myocardial infarction. Data from clinical trials in patients with acute myocardial infarction (MI) suggest that the use of bone-marrow mononuclear cells (BMMNC) may improve the left ventricular contractility and perfusion1,2. Bone marrow in adults contains various populations of cells which may have high potential to induce myocardial and endothelial repair. In order to reduce the ischaemic injury the BM cells must undergo mobilisation into peripheral blood and subsequent homing and engraftment into the target organ3-8.
This review is focused on the trafficking of stem/progenitor cells induced by the myocardial ischaemia in the setting of acute coronary syndromes.
Mobilisation of stem/progenitor cells
The pool of mobilised cells contains predominantly committed lineages such as polymorphonuclear granulocytes, T and B lymphocytes, monocytes and small population of cells which display the potential for differentiation into endothelial lineage (endothelial progenitor cells, EPC), hematopoietic progenitors (HSC), stromal (mesenchymal) cells (MSC) and other populations of putative multipotent tissue-committed progenitors5,9. It is important to remember that adult bone marrow actually contains very little “true” stem cells (self-replicating, capable of generating, sustaining and replacing terminally differentiated cell lineages). In the postnatal bone marrow the EPCs represent only 1-2% and MSC less than 0.001% of total number of BMMNC. Stem and progenitor cells are identified using the flow cytometry (FACS) by the presence of surface markers (receptors) classified according to the cluster of differentiation (CD) nomenclature. Importantly, the markers are distributed among different populations of cells and no single marker identifies the particular type of progenitor cells, so the set markers must be used instead (eg. presence of CD133, CD34, VEGF receptor 2 for identification of EPC). The confusion is even greater because the commonly used markers of stem cells (eg. CD34) can be also present on non-stem cell populations, such as mature EC and during maturation some of the markers are vanishing from the cell surface (eg. CD133 present on EPC and not on the mature EC). Therefore considerable controversies exist in the literature in regard to identification of particular progenitor cells types9,10.
Therefore the data on the mobilisation and trafficking of stem/progenitor cells in the ACS must interpreted with caution and the mechanisms and changes over time of one particular population of cells may not be extrapolated to other cell types.
Populations of cells mobilised in ACS
The most consistent data describe the mobilisation of EPC in ACS. The group of Shintani described for the first time the rapid increase of the number of CD34+ cells, identified using cell cultures and immunostaining as EPC which reached peak 1 week after the MI and coexisted with the significant increase of plasma VEGF levels. Elevated number of EPC was still found as late as 28 days after the infarct11. The increase of EPC count in acute MI and unstable angina was subsequently confirmed by other authors12. The study of Masa et al involving 26 patients with acute MI showed the significant mobilisation CD34+ stem/progenitor cells. The cell count reached peak within median 3 hours after the onset of symptoms and returned to the levels comparable with healthy subjects after 60 days. This study confirmed the mobilisation of CD34+/CD133+/ VEGFR3+ EPC as well as other poorly defined populations, such as CD34+/CD117+ cells in acute MI13. In contrast, Leone et al showed that in more than 50% of patients the peak number of CD34+ cells has been found on 5th day after infarction. The number of CD34+ was significantly lower after 1 year than in acute phase of the MI. The target population of cells measured by FACS in this study were co-expressing CD34 and CD45 (95%), CD33, CD13 and in high percentage also CD133, HLA-DR and CD38. Interestingly only approximately 50% of cells were positive for CXCR4 receptor and 1% positive for the VEGFR214.
Mesenchymal stem cells attracted attention as a potential source of cells for myocardial repair. The data regarding mobilisation of MSC in AMI is not consistent. Some studies showed changes in the number of MSC, but the significance of these findings has to be confirmed and the methods of identification of MSC refined15.
Several factors can influence the mobilisation of EPC from the bone marrow as well as the number and functional capacity of BM progenitor cells. It seems that age and the presence of diabetes are the most important factors associated with reduced number and impaired function of mobilised cells16,17.
Mechanisms of stem/progenitor cell mobilisation
Stem cells mobilisation is a part of the general inflammatory response to the myocardial ischaemia and necrosis, however experimental and clinical data strongly suggest that up-regulation of some chemokine/chemokine receptor axes are also involved in the trafficking and engraftment of stem/progenitor cells committed to induce cardiac repair18,17. Numerous hematopoietic and inflammatory cytokines are markedly up-regulated in acute coronary syndromes and may modulate the cell mobilisation (Table 1)11,13,19,20. The role of cytokines in EPC mobilisation is associated with the modulation of the binding of EPC by bone marrow stromal cells9,17. The stromal-derived factor-1 (SDF-1)/CXCR-4 axis plays pivotal role in stem/progenitor cell mobilisation and homing. The importance of this axis is evidenced by the knock-outs of CXCR4 or SDF-1 which result in a significant defect of colonisation of embryonic BM by hematopoietic stem cells and defective development of heart and large vessels18. CXCR4 receptor was identified on embryonic pluripotent stem cells, primordial germ stem cells and stem cells populations enriched in the tissue specific markers (putative tissue committed stem cells). CXCR4 expression is regulated by hypoxia via the inducible factor-1 alpha and NF-κb, proteases (cathepsin G, elastase, dipeptidylpeptidase) and down-regulation by binding to SDF-118. SDF-1 is overexpressed in response to various forms of tissue damage including myocardial infarction (infarct border-zone) and limb ischaemia. In addition the plasma SDF-1 levels are increased in acute MI (Figure 1)21,22. SDF-1 expression is downregulated by G-CSF what might explain the failure of the clinical use of the G-CSF for stem cell mobilisation in patients with the MI. Therefore SDF-1 seems to be the most important chemo-attractant for the populations of cells associated with endogenous tissue repair after ischaemic injury18.
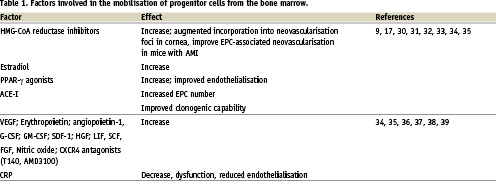
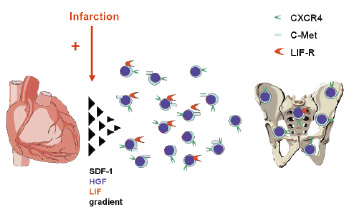
Figure 1. The circulation of cardiac and endothelial committed stem cells. The CXCR4+ cells are detectable in low number in peripheral blood in healthy subjects. Up-regulation of SDF-1, HGF and LIF in acute myocardial infarction induces the chemotaxis of cells expressing the chemokine receptors to the infarcted myocardium4,22,40,41.
The presence of CXCR4 identifies population of cells enriched in the markers of early cardiac commitment. Recently published evidence shows that adult murine bone marrow harbours non-hematopoietic CXCR4+/Sca-1+/lin–/CD45– cells expressing markers of pluripotent embryonic stem cells (Oct-4, Nanog, SSEA-1) and are capable of differentiation into all three germ layers including cardiomyocytes23. In mice it was shown that postnatal bone marrow contains cells expressing early cardiac lineage markers (Nkx2.5/Csx, GATA-4, and MEF2C). These cells are mobilised into peripheral blood in experimental myocardial infarction and the expression of cardiac and endothelial markers is markedly up-regulated. The cells express the CXCR4 as well as other chemoattractant receptors (c-met and leukaemia inhibitory factor receptor, LIFR). Importantly, the infarcted myocardium produced ligands for c-met and LIFR – hepatocyte growth factor (HGF) and leukaemia inhibitory factor (LIF), respectively as well as VEGF. The cells enriched in cardiac markers are chemo-attracted to the homogenates of infarcted myocardium in the SDF-1/CXCR4, LIF/LIFR and HGF/c-met dependent pathways. The same population of bone marrow cells in humans is likely to be identified as CXCR4+/CD34+/CD133+/CD45-22. Our data from patients with acute MI shows a significant mobilisation of CD34+/CXCR4+, CD34+/CD117+ and c-met+ cells which occurs within 6-12 hours after the onset of symptoms in comparison to healthy subjects, who had no detectable numbers of CD34+/CXCR4+ cells and only small numbers of CD34+/CD117+ cells. In addition to the mobilisation of CXCR4+ cells we found a significant up-regulation of early myocardial (Nkx2.5/Csx GATA-4 MEF2C), muscle (Myf5, MyoD, myogenin) and endothelial (VE-cadherin, von Willebrand factor) markers in the PBMNC and showed that their peak expression parallels the most significant increase of stem cells number. The concentrations of G-CSF, IL-6, HGF, SDF-1 and VEGF increased significantly in MI patients and the logistic regression revealed that, only the significant increase of SDF-1 level was an independent predictor of CD34+ cells mobilisation19. Fazel et al showed that another CD117/SCF signalling axis is crucial for the mobilisation and engraftment of CD117+ BM derived progenitor cells24.
Mobilisation of stem progenitor cells and the outcomes in acute coronary syndromes
The recently published data from the prospective analysis of patients with stable coronary artery disease (CAD) showed that mobilisation of stem/progenitor cells may have prognostic value25. So far much less is known with regard to the utility of measurement of the number of EPC and other progenitor cells in ACS as the predictors of adverse events. So far, available data suggest that the number of circulating progenitors, in particular EPC are correlated with the surrogate end-points, such as left ventricular remodelling and ejection fraction (LVEF) as well as the infarct size.
Study published by Leone et al suggest that the number of circulating CD34+ cells measured 1 year after the infarction is significantly correlated with changes of LVEF, left ventricular wall motion index and left ventricular remodelling (end-diastolic volume) changes in comparison to acute phase of the MI. The number of CD34+ cells was independent predictor of the improvement in global and regional left ventricular contractility after one year, but not in acute phase of the infarction. The primary finding of this study is that the patients who had persistently higher CD34+ cells count had also less left ventricular remodelling and better LV function improvement after one year14. Study of our group showed that the mobilisation of two cell subsets CD34+/CXCR4+ and CD34+/CD117+ cells also in the acute phase of MI is positively correlated with LVEF measured within five days after admission as well as after one year of follow-up20. The data was confirmed in the prospective observation of patients with the MI which had the cardiac MRI with adenosine stress test and late enhancement. The number of CD34+/CXCR4+ cells was positively correlated with the LVEF and negatively correlated with the number of myocardial segments displaying impaired first pass perfusion after adenosine after 1 year. The acute mobilisation of stem/progenitor was blunted in patients with reduced LVEF and high NT-proBNP26. The number of circulating CD34+/CXCR4+ cells was also inversely correlated to the area of the delayed enhancement and the levels of cardiac troponin I and activity of CK-MB suggesting that patients with more extensive area of the infarction might have impaired mobilisation26,27. The functional in vitro assays revealed significant increase of colonies derived from mobilised endothelial and haematopoietic progenitors in MI patients. In addition to the increase of EPCs numbers, their capability of endothelial maturation may also affect functional improvement and reduction of the infarct size and favourable remodelling in patients with acute MI28.
This associations are however hypothetical and may be bidirectional, because large infarction may mobilise and attract more cells early in the course of the ischaemia leading to lower number present in the peripheral blood after few hours. Also other data sets showed no significant associations between stem/progenitor cell number and left ventricular function in the MI11,13,29.
Summary
Acute myocardial infarction is associated with the mobilisation of bone marrow-derived stem progenitor cells, including cell subtypes that may be involved in endothelial and myocardial repair (EPC, HSC and MSC) as well as sloughed mature endothelial cells. Presently the data relating the mobilisation of EPC and HSC and the outcome in acute coronary symptoms are limited and large study confirming this association is needed before the cell counts would be used as a prognostic marker in this setting. Moreover, in addition to the assessment of the cell number, it may be useful to obtain the additional data such as migratory capability, viability and gene expression profile to further improve the prognostic utility. Should the data from large prospective studies confirm the prognostic utility of the circulating EPC number it would be a powerful diagnostic tool because the number and function of EPC reflect the turnover and reparatory potential of the arterial endothelium10.
Acknowledgement
This study was supported by grant PBZ-KBN-099/P05/2003 by the Polish Ministry of Education and Science (MT) and a Servier Research Grant (WW).

