Abstract
Background: Aortic penetrating atherosclerotic ulcer (PAU) is one of the causes of acute aortic syndrome. Few studies have evaluated the value of intravascular ultrasound (IVUS) imaging in the diagnosis of PAU.
Objectives: We aimed to evaluate the value of IVUS imaging in diagnosis of PAU.
Methods and results: From September 2002 to May 2005, a consecutive series of 15 patients with suspected aortic dissection underwent both IVUS imaging and spiral Computed Tomography (CT).
CT documented 4 PAUs in three patients. There were no complications related to IVUS imaging. The common IVUS features of these four PAUs appeared as a crescentic, localized, outpouching thickened aortic wall with heterogeneous echoic density that communicated with the lumen via a discontinuous intima. By using these features, IVUS detected five other PAUs in four patients, which were overlooked by CT. The width of PAU detected by CT was significantly wider than that of PAU not detected by CT (1.33±0.67cm vs 0.43±0.27cm, P=0.027). Two of five PAUs omitted by initial CT were confirmed by follow-up CT or magnetic resonance imaging (MRI). During follow up, three PAUs, including two of those overlooked by CT, developed into aneurysms.
Conclusion: IVUS imaging is a safe examination, and more sensitive than spiral CT to diagnose PAU.
Introduction
Classic aortic dissection (CAD), intramural hematoma (IMH) and penetrating atherosclerotic ulcer (PAU) are three anatomical presentations of acute aortic syndrome (AAS)1. PAU is defined as an ulceration of an atherosclerotic lesion that penetrates from the internal elastic lamina into the media2,3. With advanced imaging techniques such as CT, MRI and transesophageal echography (TEE), PAUs are diagnosed more frequently than before. However, sometimes because of their small size, the detection of PAU is challenging by these non-invasive modalities4-6.
Intravascular ultrasound (IVUS) is an imaging technique that can supply real time, cross-sectional vascular images. Several studies have shown an adjunctive role for IVUS imaging in patients with CAD and IMH7-10. However, few authors have evaluated the value of IVUS imaging in patients with PAU.
Methods
Patient population
This was a single centre, prospective, observational study. From September 2002 to May 2004, 92 patients suspected as AAS were admitted to our hospital, and the diagnosis of AAS was established in 63 of them. Among these 63 patients, 5 died shortly after admission and 20 received urgent intervention. In the remaining 38 patients, IVUS imaging was performed in 15 in order to clarify an obscure diagnosis, to supply complete information for an established diagnosis, to explain a persistent symptom or to find a clue to the rapid progression of the pleural effusion. Among these 15 patients, by CT, the initial working diagnosis was CAD in 8, IMH in 5, PAU in 1, and ascending aortic aneurysm in 1. In the present study, we will focus on the patients who were diagnosed as PAU by CT or IVUS imaging.
IVUS imaging
IVUS imaging was performed after CT scan but within one week. Before the procedure, as a routine strategy, a single-dose of 70 UI/kg, non-fractionated heparin was administered intravenously, as for fractional flow reserve assessment. Then we performed the procedure using the following steps: Firstly, the right femoral artery was punctured and a regular, 0.035- inch, “J” type guide wire was inserted into the aortic root. Secondly, a 101.5 cm long, 12F, “J” type delivery sheath kit (Convey™, Boston Scientific, EP TECHNOLOGIES™, USA), straightened by a 106.9 cm long, 9.5F dilator was advanced to the aortic root through the guide wire. Thirdly, the dilator, and the guide wire were removed, and the sheath kit was left in the aortic root, via which, a 9F, 9 MHZ mechanic IVUS probe (Ultra ICE™ intra-cardiac Echo catheter, Boston Scientific, USA) was introduced to the aortic root. Lastly, after obtaining an optimal cross-sectional aortic image, the sheath kit was kept in the aortic root and IVUS catheter was manually pulled back and IVUS images were simultaneously recorded on the videotape for subsequent analysis. All steps were performed very prudently and under fluoroscopy. After procedure, the assessing site was closed by a vascular closing system.
IVUS imaging analysis
Two cardiologists (FS and HW), who were not blind to the findings of CT, interpreted IVUS images. We adopted Alfonso’s definition for IMH by IVUS but made some slight modifications11. IMH was defined as a crescentic, focal or diffuse thickened aortic wall with layered structures separated by echolucent spaces. Because there is no available definition for PAU by IVUS, the common IVUS features of PAUs diagnosed by CT were used to define PAU. Then we used this definition to analyse all IVUS images to detect PAU. We also measured the width and the depth of a PAU by adopting the method described by Cho et al. and by using Echoplaque software (INDEC systems)12. The reliability of IVUS measurements in this context has previously been published12,13.
Spiral CT and analysis
CT scan was performed before IVUS imaging and within 24 hours from onset of symptoms. A 4-slice spiral CT scanner (Somatom Plus 4 Volumezoom, Siemens, Forchheim, Germany) was used. The examination started with conventional unenhanced CT scan, then we administrated 140 ml of non-ionic contrast agent with at least 350 mg iodine per millilitre through an 18-G intravenous antecubital catheter at a flow rate of 3.5 ml/s (Ultravist, Schering, Berlin, Germany). The delay of enhanced CT scan was calculated using Test Bolus Technique with a region of interest placed in the ascending aorta (30 ml contrast agent at a flow rate of 3.5 ml/s). Scan parameters such as tube current 300 mA, tube voltage 120 kV, and rotation time 500 ms were the same for non-enhanced and enhanced CT scan, but we used collimation 4x2.5 mm and table feed per rotation 3.8 mm for non-enhanced scan and collimation 4x1 mm and table feed per rotation 1.5 mm for enhanced scan. Scan coverage began from 2 cm above the aortic arch and continued to the iliac arteries, and scan data were acquired with the electrocardiogram (ECG) signal recorded simultaneously. Imaging reconstruction was performed using retrospective ECG gating. The acquired scan data were selected for image reconstruction with respect to a pre-defined cardiac phase. A certain R-wave delay time defined the start point of data, and reconstruction parameters were 220 mm field of view, kernel B35, 1.25 mm effective slice thickness, 0.6 mm increment. Multiple reformation in sagittal, coronal, oblique sagittal, and curved projections were generated on an independent workstation (Insight, Neo Imagery, Technologies, City of Industry, Calif, 3D-virtuoso, Siemens). Maximum-intensity projection and shade-surface display reconstruction images of target areas were also produced.
Two experienced radiologists (JFB and NB), who were not blind to the findings of IVUS imaging, performed CT analysis using standard definitions. Briefly, without contrast, IMH is defined as crescentic or circular, focal or diffuse thickening aortic wall with a higher density than blood; with contrast medium, it has the same features, but with a lower density than blood. PAU is defined as a narrow neck, outpouching, contrast filled ulceration
Statistical analysis
We performed the statistical analyses using Spss 11.5 software. Quantitative data were expressed as mean±SD and qualitative data as frequency. Data were compared by using Fisher’s exact probabilities or likelihood ratio x2 test for qualitative data and Student’s t or Analysis of variance (ANOVA) for quantitative data. P<0.05 was considered significant.
Results
Patient characteristics (table 1)
From September 2002 to May 2005, 15 consecutive patients underwent IVUS imaging and spiral CT. Six patients diagnosed as PAU by CT or IVUS were included in the present study. All of them were males and the mean age was 65±9.3 years old (range 53 to 74). Four of them had hypertension and were symptomatic. In addition to CT and IVUS, three of them also underwent TEE, MRI or aortography.
IVUS findings as compared with spiral CT (table 2)
In this experience, no complications occurred related to IVUS imaging. The assessing site was closed by a vascular closing system after removing the sheath kit. There was no need for manual compression and no hematoma occurred. Even in a very dilated aorta, IVUS could supply a good cross-sectional image of the entire aorta and most side branches. In our study, the biggest aorta diameter was 89 mm, and the detecting rate of three arch branches, celiac trunk artery, superior and inferior mesenteric arteries, and renal arteries was 100%.
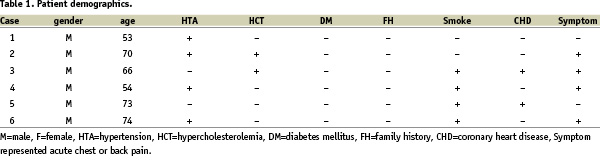
CT documented 4 PAUs in three patients. The common IVUS features of these four PAUs appeared as a crescentic, localized, outpouching thickening aortic wall with heterogeneous echoic density that communicated with the lumen via a discontinuous intima (see figure 1).
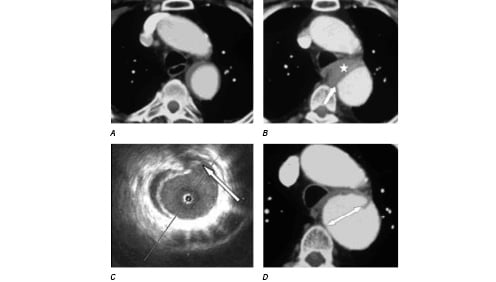
Figure 1a: IMH without evidence of PAU (CT with contrast, 2004/04/17).
Figure 1b: Enlargement of the IMH with a new onset PAU (CT with contrast, 2004/06/08, the white star represented IMH and the white arrow represented PAU).
Figure 1c: The corresponding IVUS image (2004/06/15) showed clearly this PAU (indicated by a white arrow).
Figure 1d: This PAU developed into a big aneurysm (indicated by a double head arrow, CT with contrast, 2004/08/03).
By using these features, IVUS detected five other PAUs in four patients, which were overlooked by CT. The width of PAU detected by CT was significantly wider than that of PAU not detected by CT (1.33±0.67cm vs 0.43±0.27cm, P=0.027). The depth of PAU detected by CT was greater than that of PAU not detected by CT, but the difference was not statistically significant (1.59±0.50cm vs 0.99±0.51cm, P=0.121).
In all, IVUS detected 9 PAUs in six patients (one patient had 3 PAUs, one patient had 2 PAUs, and the 4 others had 1 PAU each), and the PAUs were located in the ascending aorta for 1, in the arch for 1, and in the descending aorta in the remaining 7 cases.
Treatment and follow up information (table 2)
All patients initially received conservative therapy except one, who underwent surgical operation because of aneurismal dilatation of the false lumen. All of them were followed up by means of clinical visits or telephone interviews, and received regular CT examinations. The mean follow up time was 21.7±11.2 months (range 8-33). No deaths occurred. Two of the five PAUs that were overlooked by the initial CT scan were subsequently confirmed by follow-up CT or MRI (see figure 2).
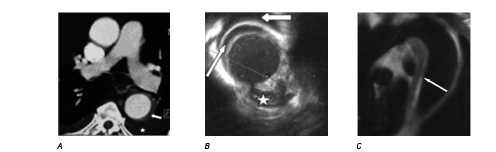
Figure 2a: IMH without evidence of PAU (CT with contrast, 2002/09/18, the white star represented pleural effusion and the white arrow indicated the IMH).
Figure 2b: IMH and PAU (corresponding IVUS image, 2002/09/18, the white star represented the PAU, the small long white arrow indicated the IMH, the big short white arrow indicated the pleural effusion).
Figure 2c: This PAU was confirmed by follow up MRI (2002/09/25, indicated by a white arrow).
Three PAUs, including two overlooked by CT, developed into aneurysms. However, there was no significant difference in width or depth between PAUs with and without progression.
Discussions
PAU was first described as a distinct clinical and pathologic entity by Stanson et al. in 19862. This condition is characterized by an ulceration that penetrates through the elastic lamina and into the media and is associated with a variable amount of hematoma within the aortic wall. Since it is not possible to differentiate PAU from IMH, CAD and other diseases by relying on clinic presentations alone, we need an imaging technique with high resolution in order to diagnose PAU. Although non-invasive imaging techniques such as: CT, TEE and MRI and invasive aortography have been reported to successfully diagnose PAU, they also have some limitations.
Several studies have shown an adjunctive role for IVUS imaging in patients with CAD and IMH7-10, however, few authors have evaluated its value in the diagnosis of PAU. Moreover, previous studies used a 20 MHZ IVUS probe, which had limitations in a dilated aorta. Theoretically, a low frequency IVUS probe can overcome this limitation. Recently, a 9MHZ IVUS probe has been made commercially available, but as yet, reports of experiences with this probe are scant. We used this new system in 15 patients over a period of three years, and our study showed that it could provide a good cross-sectional image of the entire aorta (even a very dilated one) and most of its side branches.
CT scan is presently the most frequently used imaging technique in patients with suspected AAS. By comparison with CT, IVUS imaging is invasive and requires a large femoral access (12F), but has the advantage of avoiding the use of contrast media. Up to now, only one case report has showed multiple PAUs detected by IVUS imaging14, and our study is the first to evaluate the value of IVUS imaging in a series of patients with PAU. Because there is no available definition of PAU by IVUS, among those PAUs detected by CT, the common IVUS features were used to conclude the definition, and it was defined as a crescentic, localized, outpouching thickening aortic wall with heterogeneous echoic density that communicated with the lumen via a discontinuous intima. By using this definition, IVUS detected 9 PAUs in 6 of our patients, and 6 of them were confirmed by initial or follow up CT or MRI. Therefore, our study showed that IVUS is capable of diagnosing PAU.
Our study also demonstrated that IVUS was more sensitive than CT to detect small PAUs. The mean width and depth of PAU were 0.83 cm and 1.25 cm respectively in our study, relatively smaller than the dimensions reported by others12,15,16. In addition, the width of PAU detected by CT was significantly wider than that of PAU not detected by CT. The reason why some PAUs escaped CT may be due to their small size and the limited spatial resolution of CT. According to their theoretical spatial resolutions, TEE, MRI and aortography can not do better than CT in detecting small PAUs, which was confirmed by our study. Thus, we believe that to date, IVUS imaging is the most sensitive modality in vivo for detecting small PAUs.
PAU is considered by most authors to have a poorer prognosis than CAD. Coady et al. reported that the risk of aortic rupture was considerably higher among patients with PAU (40% of cases) than among patients with type A or type B aortic dissection (7.0% and 3.6% respectively)6. Ganaha et al. reported that AIH with PAU had poorer outcome than AIH without PAU15. But some controversy still exists because the natural history of PAU is unknown, and so far there is no standard treatment strategy. In our study, all patients received medical therapy except one, who was operated on because of aneurismal dilatation of the false lumen. During follow up, three of the remaining 8 PAUs developed into aneurysms, which included 2 that had been overlooked by CT. Therefore, it is important to emphasize that small PAUs that escape CT could be also dangerous.
Clinical implications
In our clinical practice, non-invasive CT scan (4-slice spiral CT) is the first choice of imaging technique in patients with suspected AAS. MRI, TEE and aortography play as additional tools. However, sometimes even after performing all 4 of these examinations, some unsolved problems still remain, such as obscure diagnoses, persistent symptoms, rapid progression of pleural effusion etc. In these circumstances, our experience showed that IVUS imaging with a 9 MHZ probe was very helpful. This study demonstrated that IVUS imaging was very sensitive to detect PAU, particularly small ones, which may be due to its high spatial resolution. However, to date, the potential value of IVUS imaging for the treatment strategy for PAU remains unclear. We do not think that IVUS imaging can replace a non-invasive imaging technique like CT scan as the first line examination in the setting of AAS, especially with the introduction of new generation non-invasive imaging techniques (for example, a 64-slice CT scanner), which have higher spatial resolutions.
Study limitations
1. Not all PAUs that escaped CT were subsequently confirmed, thus, we couldn’t exclude false positive results. 2. Although the time interval between IVUS imaging and CT was short and there was no evidence of clinical progression, we cannot exclude possible changes during that period of time. 3. Although there were no complications in our study, IVUS imaging is an invasive examination that has potential side effects.
Conclusions
IVUS imaging is a safe examination that is able to diagnose PAU. It is very helpful in detecting small PAUs that can be overlooked by CT scan, and which may be dangerous.

