Abstract
Aims: When using bare metal stents (BMS), direct stenting (DS) has become an established method for percutaneous coronary interventions (PCI). If drug-eluting stents are used, DS is currently not recommended.
Methods and results: We analysed the data of the German Cypher Stent Registry to evaluate current use and outcome of DS using the sirolimus-eluting stent (SES). 4,437 patients at 122 hospitals who received a single SES for one lesion and completed follow-up, were included. DS was performed in 1,727 (38.9%) of these patients. However, there was a wide range from 0% to 77.8% within the centres. The following factors were independently associated with the use of DS: degree of stenosis (per 10%) (p<0.0001), type C lesion (p<0.0001), three vessel disease (p<0.0001), age (per decade) (p<0.0001), target vessel = LAD (p=0.0029), diabetes mellitus (p=0.0222) and renal insufficiency (p=0.0260). There were no higher event rates for DS compared to predilatation from admission until the end of follow-up: death rates were 1.4% versus 1.9%, p=0.1854, TVR rates 7.9% versus 8.7%, p=0.3388 and TVR or MACCE rates 10.8% versus 12.1%, p=0.2088. This was confirmed after correction for confounding factors.
Conclusions: In current clinical practice DS with the SES is performed within a very wide range at the different hospitals. Further predictors for the use of DS with the SES are similar to those known from BMS. DS with the SES in such selected patients seems to be safe and effective.
Introduction
The introduction of coronary stents1,2 has made percutaneous coronary interventions (PCI) much more predictable and safe3. Stents also reduce restenosis rates and lower the need for target vessel revascularisation (TVR)4,5.
Using the first stents that were available, predilatation with a balloon was necessary in most cases before stent implantation. Newer stent generations made it possible to directly implant stents in many cases without predilatation. By reducing the extent of vessel injury and thus creating less intimal hyperplasia, direct stenting has been postulated to reduce restenosis compared to stenting with predilatation6,7.
However, randomised controlled clinical trials, comparing direct stenting with stenting after balloon predilatation, found no reduction in restenosis and TVR rates, but fluoroscopy times as well as the consumption of contrast media could be reduced8-16.
Advances in drug-eluting stents (DES) could further reduce restenosis and TVR rates17-21. In the case of DES, direct stenting was not recommended because of the fear of damaging the drug layer by passing through a high grade stenosis and thereby potentially loosing the drug effect.
Nevertheless in clinical practice direct stenting is also used in the setting of DES and some reports suggest that it may be associated with the same results as DES implantation with predilatation22-25.
In order to determine the use, predictors and outcome of direct stenting with the sirolimus-eluting stents (SES) in patients treated with SES in daily clinical practice, we analysed data from a prospective multi-centre nationwide DES registry, the German Cypher Stent Registry26,27.
Methods
The German Cypher Stent Registry is a project of the ‘Deutsche Gesellschaft für Kardiologie’ (DGK, German Cardiac Society), the ‘Bund der Niedergelassenen Kardiologen’ (BNK, The Association of Out-Of-Hospital Cardiologists) and the ‘Arbeitsgemeinschaft Leitende Kardiologische Krankenhausärzte’ (ALKK, The Working Group of Hospital Cardiologists). It is sponsored by Cordis Corporation, a Johnson & Johnson Company. Details of the registry are described elsewhere26,28.
In brief, the aim of this prospective multi-centre registry was to monitor current use and outcomes of the SES in daily clinical practice. Inclusion started simultaneously with the launch of the SES in Germany on April 14th 2002 and recruitment of the scientific data base of the Cypher registry was finished in December 2004. Case report forms were collected via the Internet with description of the target lesion and interventional characteristics given by the implanting physician.
Follow up
A follow-up was completed at a median of 6.6 (quartiles: 6.1 – 8.1) months after the implantation of the SES. The follow-up was performed either by the data centre in Ludwigshafen or by the treating hospital. All patients were contacted via telephone. All events were verified by hospital charts or by direct contact with the treating physician. If patients could not be reached, the ‘Einwohnermeldeamt’ (local government registration office) was contacted. Completeness of the follow-up was achieved in 91.0% of patients.
Quality Assurance
A query management was established for missing or implausible data. Announced source data verification was performed at 15 randomly selected hospitals, with comparison of the documented data with the hospital charts.
Definitions
Target vessel revascularisation (TVR) was defined by either percutaneous coronary intervention (PCI) or coronary bypass grafting (CABG) of the initially treated coronary vessel. Myocardial infarction was defined as either ST-elevation myocardial infarction (STEMI) or non ST-elevation myocardial infarction (NSTEMI). The final diagnosis was left to the treating physician. Major cardiovascular or cerebral adverse events (MACCE) were defined by either death by any cause, myocardial infarction or stroke.
Only patients treated with one SES for a single lesion and a completed follow-up were included in the current analysis. Furthermore, patients treated for chronic total occlusions were excluded.
Role of the funding source
As stated above, the design of the German Cypher Stent Registry as well as the collection, analysis and interpretation of the data were all independent of Cordis Corporation, a Johnson & Johnson company, who supported the study.
Statistics
Data collection
Data were collected via the Internet by the ‘Institut für Klinische Kardiologische Forschung’ (IKKF = Institute for Clinical Research) of the German Cardiac Society. The patients gave written informed consent for processing of their anonymous data at the ‘Institut für Herzinfarktforschung’, Heart Centre Ludwigshafen.
Data analysis
Absolute numbers and percentages are computed to describe the patient population. Medians (with quartiles) or means (with standard deviation) are computed as appropriate. Categorical values were compared by chi-square test and continuous variables were compared by two-tailed Wilcoxon rank sum test. The Cochran-Armitage test was used to analyse changes in the use of direct stenting over the years. Kaplan-Meier curves were calculated for TVR and TVR or MACCE during follow-up. Backward logistic regression analysis was first used to analyse factors being independently associated with the use of direct stenting compared to stenting with predilatation. Then further analyses were done to determine if direct stenting was an independent predictor for TVR as well as the combined endpoint of TVR or MACCE. The following variables were examined: age (per decade), gender, diabetes mellitus, arterial hypertension, renal insufficiency, target vessel = bypass graft, number of diseased vessels, target vessel = left anterior descending artery, de novo stenosis, in-stent restenosis or restenotic lesions, type of lesion according to the AHA/ACC classification, ostial location of the lesion, diameter of the stent (per 0.25 mm), length of the SES (per 10 mm), and those variables with showed a difference between direct stenting and stenting with predilatation in univariate analysis with a p-value of less than 0.1.
The area under the receiver-operator characteristic (ROC) curve, as assessed by the C statistic, was used to determine the association of predicted probabilities and observed response of the logistic regression models. P-values <0.05 were considered significant. All p-values are results of two-tailed tests. The tests were performed using the SAS® statistical package, version 8.2 (Cary, North Carolina, USA).
Results
From April 2002 until December 2004, 4,437 patients at 122 hospitals who received one SES for one lesion during their index PCI, after exclusion of chronic total occlusions and who completed follow-up, were included in the German Cypher Stent Registry. Median follow-up time was 6.6 (quartiles: 6.1 – 8.2) months. Direct stenting was performed in 1,727 (38.9%) of these patients.
Direct stenting with the SES in clinical practice
Clinical characteristics, angiographic and interventional findings of direct stenting compared to stenting after predilatation are given in tables 1 to 3. Overall, patients treated with direct stenting were younger (63.4 versus 65.1 years, p<0.0001), had undergone prior coronary bypass surgery less often (12.1% versus 18.3%, p < 0.0001) and had a lower prevalence of both renal insufficiency (8.3% versus 12.3%, p < 0.0001) and diabetes mellitus (26.2% versus 31.6%, p = 0.0001). Concerning angiographic findings, they more often had single vessel disease (40.5% versus 29.4%, p < 0.0001) and less often type C stenosis according to the AHA/ACC criteria (11.7% versus 20.3%, p < 0.0001). Pre-interventional degree of the stenosis was lower (83±11% versus 88±9%, p < 0.0001), and the maximal balloon diameter for stent implantation was bigger (3.0±0.3 mm versus 2.9±0.3 mm, p < 0.0001). With the exception of a considerable lower use of glycoprotein IIb/IIIa antagonists during direct stenting (16.4% versus 22.3%, p < 0.0001), there were only minor differences concerning medication during stent implantation and medication at discharge between the two groups (Tables 3 and 4).
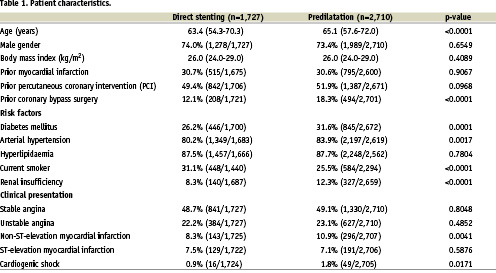

Use of direct stenting according to the different hospitals and development in the use of direct stenting
Mean use of direct stenting was 37.8±18.7% (Median: 38%, quartiles: 25% - 50%) at the participating hospitals. However, as shown in figure 1, there was a wide range within the hospitals from 0% to 77.8%. There was a small but significant increase in the use of direct stenting from 2002: 34.9% until 2004: 40%, p for trend= 0.03 (Figure 2).
+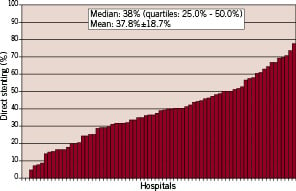
Figure 1. Amount of direct stenting with the SES in the participating centres with at least 10 patients included.
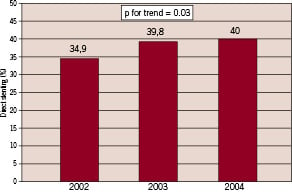
Figure 2. Development in direct stenting over time.
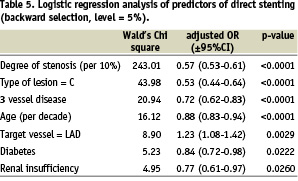
Predictors of direct stenting
Table 5 gives the independent variables which were associated with the use of direct stenting. The C statistic for this analysis was 0.69.
Outcome of direct stenting
There were no higher event rates for direct stenting compared to predilatation from admission until the end of follow-up, as shown in table 6 and figures 3a and 3b: death rates were 1.4% versus 1.9%, p = 0.1854, TVR rates 7.9% versus 8.7%, p = 0.3388 and TVR or MACCE rates 10.8% versus 12.1%, p = 0.2088.
Logistic regression analysis identified the following parameters to be associated with TVR: Target vessel = coronary bypass (OR = 2.00 95% CI: 1.36 – 2.94, p < 0.001), two or three vessel disease (OR = 2.20, 95% CI: 1.64 – 2.94; p < 0.001), diameter of the stent (per 0.25 mm) (OR = 0.88, 95% CI: 0.80 – 0.96; p = 0.005), and age (per decade) (OR = 0.85, 95% CI: 0.76 – 0.94; p = 0.004), whereas direct stenting was no independent predictor (OR = 1.06, 95% CI: 0.84 – 1.35; p = 0.61). The C statistic for this analysis was 0.64.
The following variables were associated with TVR or MACCE: Target vessel = coronary bypass (OR = 2.20 95%CI: 1.41 – 3.44, p < 0.001), two or three vessel disease (OR = 2.00, 95%CI: 1.55 – 2.56; p < 0.001), renal insufficiency (OR = 1.47, 95% CI: 1.10 – 1.95; p = 0.008), indication = STEMI/NSTEMI (OR = 1.61, 95%CI: 1.27 – 2.06; p < 0.001), reduced LV-function (OR = 1.59, 95%CI: 1.17 – 2.15; p = 0.003), diameter of the stent (per 0.25 mm) (OR = 0.90, 95%CI: 0.83 – 0.98; p = 0.011) and ostial lesion (OR = 1.35, 95% CI: 1.02 – 1.79; p = 0.039), whereas direct stenting was no independent predictor (OR = 1.02, 95% CI: 0.82 – 1.26; p = 0.87). The C statistic for this analysis was 0.65.
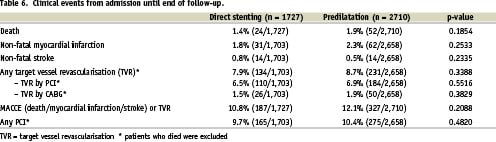
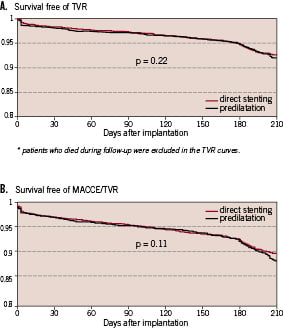
Figure 3a. Kaplan Meier curves for any target vessel revascularisation (TVR) in patients with direct stenting compared to those with predilatation.
Figure 3b. Kaplan Meier curves for MACCE (all deaths, myocardial infarctions, strokes or TVR) in patients with direct stenting compared to those with predilatation.
Discussion
Direct stenting with bare metal stents is used in about 40% of stent implantation in current clinical practice29. Its use is associated with a reduction in radiation exposure and consumption of contrast media, but not with a reduction in restenosis or TVR rates. Little is known on the use of direct stenting with SES in clinical practice.
Actual use and development of direct stenting with SES in clinical practice
In the large German Cypher Stent Registry, which included 7,445 patients from 122 hospitals, the rate of direct stenting for one lesion treated with one SES was 38.9%, if chronic total occlusion is excluded. We found a small but significant increase in the use of direct stenting between 2002: 34.9% and 2004: 40% (p for trend = 0.03).
The observed rate of direct stenting is very close to real life data with bare metal stents, such as the data from La Vecchia et al, who found a rate of 37% in clinical practice29, the 20% use reported by Wilson et al30 and the 43% reported by Herz et al31. Data from two retrospective analyses of randomised controlled clinical trials on DES looking at direct stenting reported different results. In the analysis reported by Silber et al22, the rate of direct stenting with the paclitaxel-eluting stent in the TAXUS II trial was 8.9% (23/257), whereas Schlüter et al23 on behalf of the pooled E- and C-SIRIUS trials reported a rate of 25% (57/225).
Predictors of the use of direct stenting
The most powerful predictor of the use of direct stenting was the strategy of the different hospitals and thus the preference of the treating physician. This is supported by the wide range (0% to 77.8%) of use of direct stenting at the participating hospitals, which may reflect different degrees of adherence to the recommendations not to perform direct stenting as expressed by the DES producing companies, as well as different individual experiences with direct stenting.
Factors associated with a lower use of direct stenting were: The complexity of stenoses, as expressed by a higher rate of type C lesions according to the AHA/ACC criteria and a higher, pre-interventional, degree of the stenosis. Both features are associated with a lower probability to cross a given, but not pre-dilatated, lesion with a pre-mounted stent. Similar findings were observed by La Vecchia et al with direct stenting with BMS29 as well as by Schlüter et al in the E- and C-SIRIUS trials with the SES stent23.
Other factors associated with a higher use of direct stenting were a younger age and target vessel = LAD, whereas the presence of a 3 vessel disease, diabetes mellitus and renal insufficiency were associated with a lower use. Increasing age as a predictor not to do direct stenting was also described by La Vecchia et al29 and may be due to increasing calcifications with increasing age. Calcifications by themselves make direct stenting much more problematic. More severe calcification, which may also be more prevalent in diabetic patients and patients with renal insufficiency, was also a predictor against direct stenting in the series of Schlüter at al23.
In contrast to the data of La Vecchia, who found that direct stenting was less often used in the case of ST-elevation myocardial infarction, we found no significant influence of ST elevation myocardial infarction on the use of direct stenting. Poor distal vessel visualisation due to the frequently present total obstruction and the uncertainty of the correct position of the guidewire may have prohibited direct stenting in many cases of acute ST-elevation myocardial infarction, whereas the underlying plaque rupture is easier to treat with direct stenting than a fixed calcified stenosis in stable angina. Therefore the preference and experience of the individual physician may play a special role under these circumstances.
Outcome of direct stenting compared to stenting with predilatation
Direct stenting has been postulated to reduce restenosis compared to stenting with predilatation by reducing the extent of vessel injury and thus creating less intimal hyperplasia6,7. Several randomised, controlled, clinical trials comparing direct stenting with stenting with balloon predilatation found no reduction in restenosis and TVR rates, but fluoroscopy time, as well as the consumption of contrast media, could be reduced8-16.
To the best of our knowledge there are no data from randomised controlled clinical trials with DES comparing direct stenting with stenting with predilatation. The available data from the literature22-24, together with our data, suggest that direct stenting is equivalent to stenting with predilatation concerning restenosis and TVR rates even with DES:
Silber et al on behalf of the TAXUS II investigators22 found a TVR rate at 6 months of 4.3% (1/23) for direct stenting compared to 6.2% (14/227) for predilatation with the paclitaxel-eluting TAXUS stent (p=1.00). The rates for percent diameter stenosis in the analysis segment were: 4.3% versus 4.8%, p=1.00.
Moses at al24 reported an 8 months binary in-lesion restenosis rate of 6.0% in 225 patients with direct stenting compared to a historical control group (412 patients) of 9.1% with predilatation using the SES stent (p=0.30).
Schlüter at al on behalf of the E- and C-SIRIUS trials investigators23 found no differences in in-lesion binary restenosis rates at 8 months (direct stenting 2.0% versus predilatation 6.1%, p=0.46) as well as for target lesion revascularisation rates at 1 year (1.8% versus 5.4%, p=0.46) in 225 patients treated with the SES stent.
Our data showed a target vessel revascularisation rate of 7.9% for direct stenting compared to 8.7% with pre-dilatation (p=0.3388). Logistic regression analysis demonstrated direct stenting to not be an independent predictor (OR=1.06, 95% CI: 0.84 – 1.35; p=0.61) of lower TVR rates. The C statistic for this analysis was 0.64. This is an only moderate value, suggesting that relevant variables may not have been considered.
Conclusions
Although not recommended by the DES producing companies, direct stenting with the SES is used in about 38.9% of stent implantation in current clinical practice. The main determinant to do direct stenting is the decision of the treating physician or hospital strategy. Other reasons are less complex stenoses with a lower pre-interventional stenosis grade as well a lower age of the patient. Acute and 6 month clinical outcome of direct stenting with the SES in such selected cases seems to be equivalent to SES implantation with predilatation. However, adequately powered, randomised controlled clinical trials on this issue are needed to definitively answer this question.
Limitations of the study
This non-randomised clinical trial faces all the problems of registry data that concerns the possibility of unrecognised confounding. Therefore, adequately powered randomised controlled clinical trials on this issue are needed to definitively answer the question of whether or not direct stenting with the SES is equally effective as is SES implantation with predilatation.
Furthermore, our data solely reflect the use and outcome of the SES and therefore might not be applicable for other DES. This is especially true because of the big differences in the techniques by which the drugs are fixed on or attached to the stent32,33.
Appendix
Organisation of the German Cypher Stent Registry
Members of the Steering Committee
Christian W. Hamm (Chairman), Tassilo Bonzel, Malte Kelm, Benny Levenson, Christoph A. Nienaber, Gert Richardt, Georg Sabin, Jochen Senges, Ulrich Tebbe, Thomas Pfannebecker (Cordis), Wolfgang Witsch (Cordis)
Internet data acquisition (Institute of Clinical Research of the German Cardiac Society)
Thomas Fetsch, Petra Kremer
Statistical analysis (Institut für Herzinfarktforschung, Heart Center Ludwigshafen)
Steffen Schneider
Study coordination
Thomas Fetsch
The participating centres are reported elsewhere (26).

