Introduction
Functional mitral regurgitation (FMR) is a ventricular disease characterized by mitral valve insufficiency in the absence of structural abnormalities of the valve leaflets or sub-valvular apparatus.1 It commonly provokes heart failure and frequently confers an increase in patient mortality and morbidity. Medical therapy has a limited role for management of FMR. When treated, FMR is currently managed exclusively through open heart surgical techniques.
Background
FMR may occur either in the presence of non-ischaemic dilated cardiomyopathy or in patients with regional wall motion abnormalities due to underlying ischaemic heart disease. More specifically, FMR results not only from annulus enlargement with separation of the mitral leaflets, but also from geometric distortions of the entire valvular apparatus caused by dyskinetic or akinetic wall segments and left ventricular dilatation resulting in chordal tethering as the papillary muscles displace from their normal positions. (Figure 1)
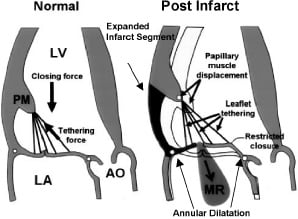
Figure 1. Left, normal mitral leaflet coapation during systole. Right, papillary muscle displacement and chordal tethering following an inferobasal MI. LA-left atrium; Ao-aortia. Modified and used with permission from Liel-Choen et al9, Copyright 2000, American Heart Association, Inc.
Furthermore, FMR itself promotes left ventricular remodeling and progressive annular dilatation in turn provoking worsened valvular incompetence.
In the case of FMR which is ischaemic in origin, coronary revascularization alone is generally considered an inadequate solution.2 Currently, surgical options for FMR utilize either replacement valves or mitral valve annuloplasty repair techniques. Replacement valve therapy suffers from risks inherent to surgery and issues related to either valve durability or risks associated with chronic anticoagulation. Alternatively, results from annuloplasty repair are often disappointing, primarily for two reasons: 1) the surgery is invasive and once again comes at an increased risk, especially in patients with advanced heart failure and 2) treatment is focused on modification of the valve annulus only. Recent publications have concluded that lack of an integrated therapy for all components of FMR including the annulus, leaflets, and left ventricular chamber has led to frequent observations of recurrent mitral regurgitation. Even just months after surgical ring annuloplasty, recurrent MR can be documented in 30% or more of these patients.3-6
To address these concerns with current mitral repair methods, a safe, more effective and easily applied therapy for FMR is needed. In addition to constraining the mitral annulus, more comprehensive treatments for patients with FMR should provide an enhanced geometric effect on the remodeled left ventricle, mitral valve apparatus and sub-valvular structures.7 Two novel technologies developed to offer more comprehensive solutions for FMR are the surgical Coapsys® and percutaneous iCoapsys mitral valve repair systems. Both approaches offer less invasive strategies for the treatment of FMR. These devices reduce the anterior to posterior dimension of the mitral valve annulus and stabilize sub-mitral valve structures (e.g. papillary muscles) to provide a more complete and long term solution to the combined annular and ventricular deformities present with FMR.
Coapsys device
The Coapsys device has been developed to treat FMR using a simplified, less invasive surgical approach. Proposed benefits include 1) off pump implantation, 2) lack of need for atriotomy, 3) ability to favourably affect and stabilize the papillary muscles and adjacent remodeled wall segments as well as the mitral annulus.
The Coapsys device (Figure 2) is composed of epicardial posterior and anterior pads which are connected by a flexible transventricular chord made of braided polyethylene.
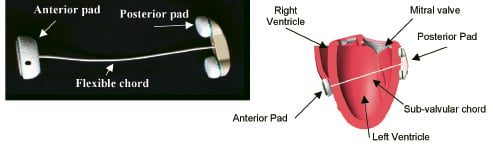
Figure 2. Coapsys device: Actual undeployed device (left) and schematic of an implant on a heart model (right).
The chord passes between the papillary muscles in a subvalvular location. Implantation occurs through a median sternotomy on a beating heart. In ischaemic FMR patients, implantation can be performed after off pump coronary bypass grafting as part of a combined procedure. Coapsys positioning is guided by external landmarks and epicardial 2-D echocardiographic visualisation of intracardiac structures. Posteriorly, the chord is introduced approximately 2.5 cm below the atrioventricular groove and midway between the papillary muscles. Anteriorly, the chord exits the base of the right ventricular outflow tract approximately 2 cm on the right ventricular side of the left anterior descending coronary artery. The chord is placed using a specially designed delivery instrument. Tailored sizing is performed intra-operatively by drawing the anterior and posterior pads together watching for significant MR reduction or elimination with transoesophageal echocardiography.
In June 2002, the TRACE (Treatment of functional mitral Regurgitation without Atriotomy or CPB clinical Evaluation) study was initiated to evaluate the procedural feasibility and efficacy of the Coapsys device for the treatment of FMR in patients undergoing Coronary Artery Bypass Grafting (CABG). Escorts Heart Institute and Research Centre in New Delhi, India was the primary participating center. The study included patients presenting for surgical CABG with grade 2, 3, or 4 FMR and a left ventricular ejection fraction of greater than 30%. The primary study endpoint was reduction in mean MR grade at 12 months measured by transthoracic echocardiography. Patients with preoperative structural abnormalities of the mitral valve apparatus such as rheumatic heart disease, leaflet prolapse, chordal rupture, mitral annular calcification and calcified leaflets were excluded from the study. Patients who demonstrated intra-operative reduction in MR grade to <2 following CABG, despite haemodynamic challenge, were not implanted with the Coapsys.
Thirty-four patients were implanted with the Coapsys device. One year clinical and echocardiographic follow-up has been reported in the initial 13 patients8 allowing the authors to conclude the following in this series of patients:
• Coapsys significantly reduced functional mitral regurgitation.
• Coapsys was associated with improved New York Heart Association (NYHA) functional class.
• The effect of Coapsys on MR reduction and NYHA class improvement was sustained at 1 year.
Figure 3 depicts these data from the initial 13 patients which have thus far become available for analysis at the one year time point.
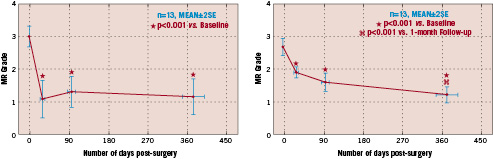
Figure 3. Graphs for the one year echo (left) and clinical (right) follow up after Coapsys implantation now reported in the initial 13 patients from the TRACE trial. (As presented in an oral abstract at ACC 2005)
Note the preservation in MR reduction and NYHA functional class over the one year duration of follow up.
iCoapsys device
The iCoapsys system is designed to provide a catheter-based percutaneous approach to performing mitral valve repair in patients with FMR. The therapeutic objective of the iCoapsys is to achieve similar reductions in MR as the surgical Coapsys device by achieving comparable annular and left ventricular shape change.
The i-Coapsys device is delivered percutaneously via the pericardial space and is composed of three major components, the anterior and posterior securement pads and the septal lateral (SL) deflector which are connected in series by the sizing chord (Figure 4).
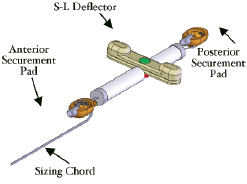
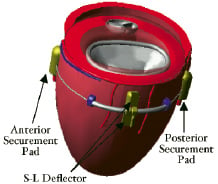
Figure 4. iCoapsys device: Diagram of the device (left) and schematic of the implant on the epicardial surface (right).
It is implanted on the epicardial surface of the heart in three stages, i.e. Access, Delivery and Therapeutic Sizing.
Access
Pericardial space access is obtained from a percutaneous subxiphoid approach. A specially designed access needle is used with fluoroscopic assistance. This access needle is fitted with a very short tip for puncturing the parietal pericardium. It also incorporates a flat base just proximal to the tip which provides depth control to prevent advancement into the myocardium. A distinct tactile “pop” can be felt as the needle punctures the parietal pericardium and enters the pericardial space. A guidewire is then advanced through the needle into the pericardial space. An access sheath is delivered over the guidewire into the pericardial space through which the iCoapsys device will be implanted.
Delivery
Coronary angiography is utilized for establishing anatomical landmarks for placement of both the anterior and posterior securement pads. The posterior pad and sizing chord are delivered on a steerable catheter through the subxiphoid access sheath into the posterior pericardial space. The posterior pad is then secured to the epicardial surface, adjacent to the PDA and approximately 2 centimeters apical to the AV groove guided by selective right coronary angiography (left coronary angiography if left dominant). Both the anterior and posterior pads are secured to the epicardium by way of deployable myocardial attachment clips controlled from the proximal end of the delivery catheter. After posterior securement has been achieved, the pad is released and its delivery catheter is removed while the pad remains attached to the long sizing chord. The SL deflector is then positioned on the posterolateral left ventricular surface by advancing its delivery catheter over the sizing chord through the access sheath. The SL deflectors final position will be determined after the anterior securement pad is placed under fluoroscopic and coronary angiographic guidance. Finally, the anterior securement pad is introduced over the sizing chord with another delivery catheter. Selective left coronary angiography is then used to position the anterior pad adjacent to the LAD. As with the posterior pad, the anterior pad is secured to the epicardial surface, and its delivery catheter is released and removed. The posterior pad, the SL deflector and the anterior pad now are attached to the sizing chord in series on the epicardial surface of the heart. A cine fluoroscopic image of the implanted device is seen in Figure 5.
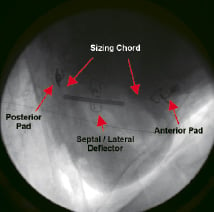
Figure 5. Cine fluoroscopic image of an implanted iCoapsys device on a live sheep model.
Therapeutic sizing
Transoesophageal echocardiography is used to optimally position the SL deflector just below the AV groove and centred on the mid region of the posterior annulus and left ventricular surface. The anterior and posterior securement pads are then drawn together by way of the sizing chord until the MR is significantly reduced or eliminated. The sizing chord is then trimmed and the pericardial access sheath removed to complete the procedure.
iCoapsys feasibility and efficacy has been demonstrated in paced canine models of FMR, human implants have not yet been performed. Echo determined pre- and immediate post- procedure mean MR grade derived from 8 canine models that underwent device implantation were 3.2 and 0.7 respectively (Communication with Myocor). Colour flow echoDoppler images of an implanted canine model can be seen in Figure 6 pre- and post- therapeutic sizing.
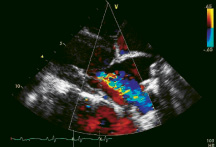
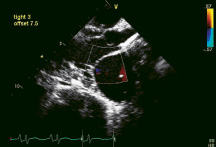
Figure 6. Colour flow echo Doppler images of a paced canine model implanted with the iCoapsys device demonstrating 4+ FMR pre-sizing (left) and elimination of FMR post-sizing (right).
Conclusion
FMR is a commonly occurring condition, especially in patients with ischaemic heart disease. It is largely due to geometric distortions of the mitral valve apparatus secondary to altered left ventricular shape and impaired function. Conventional surgical approaches are predominantly restricted to the mitral annulus and do not address the LV and papillary muscle geometric deformities intrinsic to FMR.
The iCoapsys device is designed to achieve the demonstrated advantages of the surgically delivered Coapsys system in reducing MR by way of reshaping the mitral valve annulus and buttressing the posterior myocardium in the negatively remodeled left ventricle. In addition, it offers the significant benefit of delivering an epicardial implant through a catheter based, percutaneously delivered system.

