The history of the first stent is long lost in the memory of humanity. It probably occurred at some unclear epoch when man became conscious of his own vessels, especially those that opened toward the exterior. Most likely a painful experience occurred that was caused by anomalies of these vessels, and these people divined, not yet the causes, but the mechanisms of what was going on and finally sought relief. Suffer first, understand why and then finally treat with the means at hand.
It has been said that the Egyptians already took this essentially medical step in treating the contraction or the shrinking of the urethra through the introduction of a small reed in the urinary canal. They thought that by doing this they could re-establish the relationship between fluids in the interior and exterior worlds; in a certain sense creating an easier exchange between man and nature.
True or false, this story is instructive and points us towards four questions:
– Did our ancestors evaluate the benefit-risk ratio?
– Did they eventually observe the budding of this organic stent placed in the interior of the urethra and describe this unfortunate phenomenon as a restenosis?
– Did they develop an industry and commerce in reeds for medical ends?
– Why did the organic urinary stent long ago disappear?
Today we no longer place organic stents in the urethra because for a long time Egyptians and other people have discovered better solutions, but we do place metallic stents in the coronary arteries. We can imagine that our method is identical with that of our ancestors. First there is suffering, followed by a description of angina in the chest. Then we come to understand myocardial ischemia, observing the anomalies between the coronary lumen by coronagraphy, get around the stenosis by by-pass, erase it using angioplasty and finally place the stent. In relation to this long diagnostic and therapeutic approach developed over many centuries by the passage of time and knowledge, the placement of the first stent appears as a simple step, natural and logical, in the evolution of the treatment of coronapathies. In this way the actual role of the first “stenter” appears modest, and, in any case infrequent and circumstantial. The first stent belongs to a logic of therapeutic progress initiated by Charles Dotter and Andréas Grüntzig. Both are the veritable inventors and conquerors of the interventional cardio-vascular world. They opened the way to Interventional Cardiology, a road which numerous cardiologists, radiologists and scientists have trodden since the end of the 1970s, dedicating themselves to conceiving the first stents and rapidly running into the problem of their placement in the endo-arterial space.
The story of the first endo-coronary stent is one of instruments, a team and a patient...
One day two Swedes meet. The first, Wallsten, was the inventor of a revolutionary machine destined to produce paper, the other, Senning, was a renowned vascular surgeon. Senning, the surgeon explained to Wallsten the inventor, about the seriousness of aortic dissection. He then went on to speak of his idea involving the mechanical support for the vessel wall internally by the implantation of a metallic grilled tube. Wallsten gave up paper production to dedicate himself to the development of these endo-prostheses. He created the Wallstent, and later found the solution that allowed its percutaneous implantation in a foreign body. Imbert, who completely understood the mechanical properties of the prepuce, came up with the solution (Can anything be clearer than how the genital-urinary pathways have long been of great inspiration for Interventional Cardiologists?). The self-expanding stent was thus created which could be put in place using a percutaneous peripheral arterial approach. Looking to further experiment, Imbert met Sigwart in Lausanne, as well as our team at the University Hospital in Toulouse.
We began working with the radiologists F. Joffre and H. Rousseau, beginning rapidly (perhaps too rapidly!) experimenting on sheeps and dogs. These trials proved that it was easy (perhaps too easy!) to pose a stent percutaneously, confirming the rapid development of a neo-endothelial covering of the mesh of the stent. These experiments did not allow us to measure the risk of the thrombosis of this foreign body – and to tell the truth, we underestimated that risk.
The first endo-coronary stent was implanted in man during the month of March, 1986. The history of this first patient had been banal: 63 years of age, arterial hypertension and a symptomatic restenosis 6 months after dilatation by balloon angioplasty of the second segment of the anterior interventricular artery. 1986! Evidenced based medicine was taking its first baby steps, and patients weren’t yet receiving either platelet aggregation inhibitors or statins. The procedure was done using a sub cutaneous heparin treatment during implantation, followed by the same treatment for a period of six weeks. More than nineteen years later, the patient is 83 and escaped on-stent thrombosis or intra-stent restenosis. He did not escape however, the evolution of the atheromic process which was responsible for two additional repeats of angina. The first, related to the development of a stenosis on the ostial interventricular artery was treated by a directional atherectomy in 1995, the second, on the proximal circumflex, was treated by implantation of a stent in 2004. In the weeks following this first case, seven other patients benefited from the implantation of a self-expanding stent without complications. 1986! Rules protecting patients were not yet formally in place here in France, yet still, I believe, these patients were truly respected. It was a time when patients, still little challenged or solicited by the din of the technological developments in medicine, granted their confidence more to the angio-plastician than to the angioplasty itself, with or without a stent.
These initial results were promising. They fooled us however because four of the patients presented with a precocious thrombosis of the stent1. Using only subcutaneous heparin therapy, the rate of stent thrombosis was prohibitive. Soon after Sigwart made his first implantations using a heparin anticoagulant treatment during the procedure, followed by long-term Coumadin treatment afterwards. This medical treatment allowed a reduction in intra-stent thrombosis, but which still remained high at around 5 to 10%.
These two initial clinical trials conducted first in Toulouse and then in Lausanne proved the feasibility of the technique and above all demonstrated the strong potential for thrombosis of a foreign body. We needed to wait another ten years to elaborate a platelet aggregation inhibitor treatment that allowed us to minimize the risk of thrombosis to a level that was clinically reasonable and prove the superiority of the stent over the sole use of dilatation in the prevention of restenosis2-5. From then on the stent could develop without constraint, and even, today, appear, no longer “bare” but a little... dressed.
Concerning the evolution of medicine, its hazards and necessities, this story of the first stents can inspire us to certain reflections:
– *The first “stentors” found themselves in the right place at the right time. They were but the instrument of a natural and irresistible evolution. They were, above all, modest.
– *Today, the stent occupies the entire anatomical and functional domain of coranopathies. And still, its validation, notably medical-economic, remains very incomplete. The interventional cardiologist is invited, after having spent years in learning and perfecting his or her skills, to learn not use them (to learn “le savoir faire”, and then learn “savoir ne pas faire”).
– *Coronary stenosis may be conquered, but the fight against atherosclerosis has not been won. The solution needs to be found in the direction of pharmacological athero-stabilisation and in prevention. Remember, it has been a long time since we treated complications caused by bilharziosis by placing small reeds in the urethra.

Figure 1. The future of the “stented” segment at 6 weeks
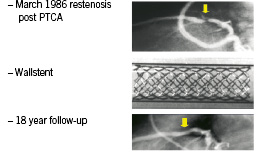
Figure 2. First coronary stenting implanted in human.

