Abstract
In the era of drug-eluting stents that have dramatically reduced the re-stenosis rate, and in the context of the development of emerging modalities like magnetic resonance imaging (MRI) and multislice computed tomography that allow precise and non-invasive diagnosis, it becomes important to question whether and how fluoroscopic-based evaluation of cardiac structure and function shall evolve. Indeed, this widely used technique is also known for its invasiveness, risk of ionising radiation effects, and projection imaging limitations.
In order to answer this question it is important to understand the technological advances under development for fluoroscopy and link them with the unmet clinical needs of today in the field of both diagnostic and interventional coronary procedures. It can then be understood how fluoroscopic-based technologies can meet these needs. This discussion shall review the emerging technologies available in the cath lab and, in doing so, portray a concept for the next generation catheterisation laboratory.
Abbreviations and acronyms
CT: computed tomography
MRI: magnetic resonance imaging
FPD: flat panel detectors
Cath lab: catheterisation laboratory
II: image intensifier
DQE: detective quantum efficiency
IVUS: intravascular ultrasound
QCA: quantitative coronary angiography
CTO: chronic total occlusion
LCx: Left Circumflex
Introduction
X-ray imaging has been used for over a century to visualise structures within the body and is the oldest diagnostic medical imaging modality. Nevertheless, this imaging modality continues to evolve and retains a pivotal role in interventional cardiology. The basic principle has remained unchanged since its introduction: an electrical source produces an x-ray beam that projects a shadow of the exposed tissues onto a display device. However, in recent years, major technological advances have been made in the fields of detector technology, image processing algorithms, storage and transfer capabilities. Lately, angiography has evolved to real-time two-dimensional display on digital flat panels (DFP)1 and more recently to three-dimensional reconstructions from rotational single plane acquisitions2.
In parallel with the technical evolution of x-ray based imaging capabilities, newer even less invasive means of imaging cardiac structures are also emerging at a rapid pace. Among these are computed tomography (CT) scanning, including multi-slice CT3, magnetic resonance imaging (MRI) and real time 3D ultrasound (US). The development of these new modalities is aimed at providing less invasive means of diagnosing structural and functional pathologies of the heart chambers and coronary arteries to replace the more invasive coronary angiography.
As such alternatives continue to be developed for diagnostic purposes and are indeed expected to assume increasingly important roles, percutaneous procedures performed in the cardiac catheterisation laboratory (cath lab) have become increasingly therapeutic in nature. Now, more than ever before, clinical decision-making is largely based on the rapid interpretation of displayed images. Consequently the demand for the highest possible image quality has never been so important. In addition, ease of use also becomes an important factor for achieving time-efficient procedures while minimising radiation exposure to patients and cath lab personnel.
This brief review shall provide an overview of current efforts to advance fluoroscopic-based imaging technology in order to meet the unmet clinical needs envisioned in the cath lab. In so doing, we provide one concept for the next generation cardiac catheterisation laboratory.
New trends in angiographic image processing
Enhanced image quality
Percutaneous procedures performed in the cath lab are becoming increasingly therapeutic in nature, calling for better image quality to allow safer and quicker procedures. In this regard several recent hardware and software improvements have been made.
Digital flat panel (DFP) technology provides enhanced image quality thanks to increased signal-to-noise ratio and wider dynamic range up to 14 bits as compared to conventional image intensifier-based detectors (II) that used only 8-10 bits4. Moreover, the perfectly flat panels are free of geometric distortion. Detective quantum efficiency (DQE) is considered by imaging physics experts to be the best characterisation of the performance of an x-ray imaging detector. It combines radiation dose to the detector, spatial resolution performance and total image noise (grainyness) into a single function. DQE has been reported to be higher with DFP than with prior conventional systems5, allowing the same image quality to be achieved at lower radiation exposures or better image quality at the same dose6.
Together with this technological revolution, the cath lab manufacturers have improved their whole image chain. For instance, GE Healthcare Technologies (Waukesha, WI, USA) claims an optimisation of the imaging components from acquisition to processing to enhance certain attributes in stent images. This involves: i) enhancing DQE at frequencies of stent struts, ii) optimising contrast to noise ratio dynamically to maintain stent visibility, iii) providing consistent visualisation of objects of varying background by a dynamic range management algorithm, and iv) spatio-temporal filtering for optimum stent visibility, as illustrated in Figure 1. This real-time approach is automated and does not involve operator intervention.
A second approach proposed by Philips (Andover, MA, USA), called ‘StentBoost’ consists of freezing and enhancing a region of the image around the stent. An algorithm sums the different frames of a dedicated record run, in order to lower the noise level while ‘boosting’ stent struts. A similar post processing developed by GE Healthcare Technologies (Waukesha, WI, USA) is illustrated in Figure 2.
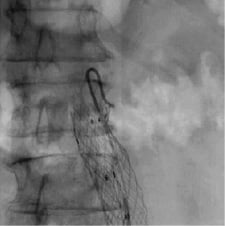
Figure 1. Illustration of the image quality offered by GE Healthcare flat panel on an aortic stent.
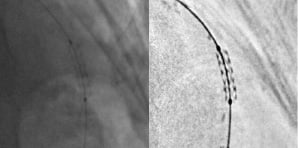
Figure 2. Illustration of stent enhancement: before (left) and after (right) post-processing.
Together with improved image quality, ease of use also becomes an important factor for achieving time-efficient procedures while minimising radiation exposure to patients and cath lab personnel. This includes automatic exposure control techniques, and the need for improved gantry manoeuvrability. For instance, non-contact capacitive sensor technology now permits the detector to be positioned automatically and quickly near the patient, without compromising safety. Image quality benefits from this automatic optimal positioning of the receptor close to the patient, as magnification and scatter radiation are reduced. Moreover, skin dose is minimised8. New cath lab generations will include even more precise dose monitoring, by tracking the radiation delivered locally to patient skin. Alert mechanisms will prevent from too long exposures of the same area, and allow patient follow-up when a skin burn is potential after a complicated case.
Three dimensional reconstructions
Angiography suffers from the intrinsic limitations of projective 2D images, and does not offer a perfect volumetric visualisation of arteries. Single plane rotational acquisition techniques provide, with a single contrast injection, a wide range of angulations for improved visualisation of the coronary arterial tree, including a 3D effect without over- or underexposure phenomena due to lung or bone superimposition. This allows better assessment of the vascular morphology and luminal narrowing, and of cardiac function2. Moreover this can be done at lower net dose of both x-rays and contrast media, while providing comparable diagnostic information to that of several fixed-angle acquisitions2. This technique can be applied to image a deployed stent after inflation while the guide wire is still in place in the stent with the balloon deflated. A tomographic algorithm is then used, and Figure 3 illustrates the potential resultant image. With slightly improved resolution this tool has the potential to enable the cardiologist to assess very accurately stent deployment, and since the balloon is still in place, offers the advantage of a potential corrective action if under-deployment is observed.
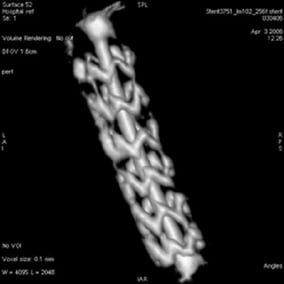
Figure 3. Illustration of a stent 3D model obtained from a GE Healthcare Spin acquisition and reconstruction algorithm in a swine model. For better visualisation, a video is available on the on-line version of the article.
Lumen morphology assessment, like stent imaging, can suffer from viewpoint orientation and from the lack of precision of quantitative measures due to magnification factor uncertainty. Here again, 3D reconstruction of the coronary arteries can lead to higher accuracy and precision in lesion severity assessment.
Different approaches to achieve 3D reconstruction of the coronary arterial tree are being investigated. One approach, available commercially from Paieon Inc. (New York, NY, USA), models vessel geometry using a small number of views (typically two or three) at different angulations of a same vessel9, (Figure 4). Geometric triangulation enables computation of the 3D vessel central line. QCA analysis in each view is then used to fit a parametric model along this central line. This approach has the main advantage of being easy and quick to use, especially to assess lesion length and to eliminate foreshortening effects. One limitation of this approach, however, is that the geometric morphology accuracy is approximate being derived from only a few angulations. In the case of a geometrically eccentric plaque, as in the case of a bifurcation, this representation can be misleading.
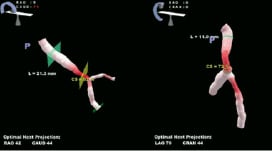
Figure 4. Illustration 3D model of a bifurcation obtained with the CardiOp-B system from Paieon Inc. in two different angles.
A second approach relies on a full tomographic reconstruction of the vessels from a rotational acquisition. In the case of the coronary arteries, motion due to respiration and cardiac contractions have to be compensated through highly specified and computationally intensive algorithms10. This challenges the clinical applicability of such approaches in the context of cardiac CT being developed at a rapid pace. Nonetheless, image processing methods are evolving quickly and 3D information from cath lab images will certainly be available soon (Figure 5).
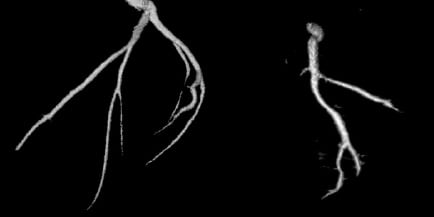
Figure 5. Illustration of coronary 3D reconstruction from a GE Healthcare spin acquisition on a swine model.
For peripheral vessels, on the contrary, the situation is easier because movement does not have to be compensated for and high-resolution images can be achieved, as shown in Figure 6. In this case, 3D information available in a couple of seconds would be valuable, for example, in tortuous carotid lesions without compromising workflow in the cath lab.
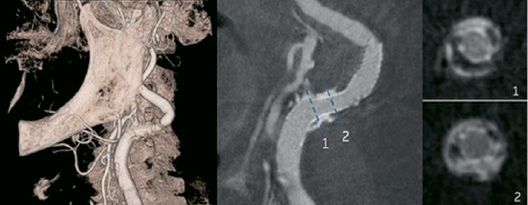
Figure 6. Illustration of a carotid 3D model from a GE Healthcare spin acquisition. Left: on peripheral vessels, 3D model with high quality images. Middle and Right: After stent placement, it is possible to observe the relationships between stent, lumen, soft and calcified plaque.
Perfusion imaging
Great achievements have been made recently to improve outcomes of patients with acute myocardial infarction. Angiographic assessment of blood flow in epicardial coronary arteries has played a pivotal role in the evaluation of reperfusion. However, clinical outcomes depend mainly on the restoration of tissue perfusion, which can be compromised by distal embolisation. The improved image quality achieved with DFP offers the possibility to quantify changes in myocardial radio-opacity during a contrast injection (frequently referred to as myocardial “blush”) for estimation of the myocardial perfusion11. Digital subtracted angiography can help the reading of arteriograms by removing background structures, as commonly done for peripheral vessels. A full non-injected heart cycle is required to be synchronously subtracted as a dynamic mask from the next cycles (see Figure 7).
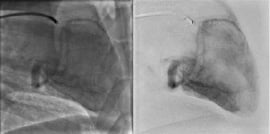
Figure 7. Illustration of Cardiac DSA in a sheep model. Comparison of subtracted images (right column), with standard images (left column).
Further steps will consist in quantifying the blush level and appearance. Similar to nuclear medicine, it becomes possible to visualise the physiological significance of a lesion by considering the dynamic behaviour of the contrast medium density before and after injection of vasodilator, before and after revascularisation. Ultimately, imaging technique could allow physiological assessment of lesion severity right in the cath lab, as fractional and coronary flow reserve (respectively known as FFR and CFR) can do.
Integration with other modalities
Intravascular imaging
Coronary angiography provides no data about the composition of intramural plaque, nor about vessel remodelling that occurs with atherosclerosis so that many vessel segments that appear normal may contain highly vulnerable regions that could lead to significant morbidity and mortality12.
Intravascular imaging offers very useful information regarding vascular anatomy and function as well as plaque composition. The most widely used approach is currently IVUS (Figure 8), but one may recall thermography and palpography, aiming at drawing a map of the vessel wall temperature and elasticity. Now under development and evaluation are optical coherence tomography (OCT)13, and intravascular magnetic resonance14. These modalities are further noteworthy in the context of this review in that it is apparent that new cath labs need to permit the practitioner to seamlessly obtain and integrate these complementary images with standard fluoroscopic images. In particular, these new imaging modalities rely on accurate, high quality fluoroscopic images for catheter positioning and arterial mapping. A good example of such integration is the co-development of an IVUS console (Volcano Corporation, Rancho Cordova, CA), directly accessible on a table user interface (GE Healthcare Technologies, Waukesha, WI, USA). The next step of integration, is the collocation on both IVUS and angiographic images of a marker, allowing the operator to visualise the corresponding lumen view directly by clicking on the desired location in the x-ray image, or on a 3D model of the vessel, as illustrated in Figure 8.
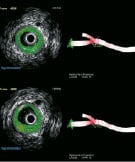
Figure 8. Illustration IVUS and Virtual Histology™ images from Volcano Corporation, registered with a 3D model of a bifurcation obtained with the CardiOp-B system from Paieon Inc. Virtual Histology™ IVUS uses advanced software to provide coloured images of the plaque in the arteries in addition to the conventional grey scale images. The top line shows the lumen, proximally to the bifurcation, while the bottom line shows the beginning of the bifurcation.
Fusion with three dimensional data
The combination of 3D models of the heart, vessels or chambers with fluoroscopy may have significant clinical implications in any procedure requiring a real-time visualisation of heart anatomy, allowing for more accurate localisation and dynamic positioning of new classes of interventional devices. For instance, several different approaches are being developed for percutaneous aortic valve replacement, mitral annuloplasty, atrial septal defect closures, coronary bypass procedures and ablations for atrial fibrillation. These new procedures shall be facilitated by the ability of the clinician to identify and accurately manoeuvre catheters within the coronary sinus and venous system, to understand the anatomic relationships between epicardial arteries and veins, to perform atrial transseptal procedures, to manipulate catheters within the left ventricular cavity (particularly under the valve cusps) and to manipulate catheters within the left atrium15. Depending on the need, various modalities can provide such 3D information, among these of course being CT, MRI and 3D Echo. Each modality has features which shall render it optimal for specific applications. The integration of these modalities with real time angiography can be achieved at different levels. First, it must be possible to review the acquired image during the interventional procedure. This shall be facilitated through normalisation of image format between the different image modalities and among device manufacturers. But, as the cardiologist is concentrated on his patient, it would be very useful to have the capability to review 3D data through the angiographic interface. GE Healthcare has lately proposed this, through the display of a CT cardiac volume on one of the in-room monitors. The novelty of this approach is that the CT volume is displayed synchronously with gantry movements. This feature could have interesting benefits, for example, during the crossing of chronic total occlusions when no contrast dye can get through the occluded artery. The CT volume oriented in the same way as the angiographic image would then be used as a reference image. In-lumen 2D views would aid in understanding lesion composition3, while comparison or co-registration of the projected 3D luminal view with the 2D x-ray image could provide guidance to navigate through the lesion.
A further advance in the integration of the imaging modalities is the actual fusion of two different images. One way to accomplish the needed registration is to use a geometric transformation that aligns points or anatomic features16. In patients in whom registration is performed shortly after CT scanning, it is unlikely that the anatomy of the organ, such as the left atrium, will change. It can thus be assumed that the imaged and registered anatomic structure will behave as a rigid body. A complete illustration of this approach in presented in Figure 9, during a left atrial ablation procedure, for the treatment of atrial fibrillation. Similar approaches will likely find application in the other applications noted at the beginning of this section.
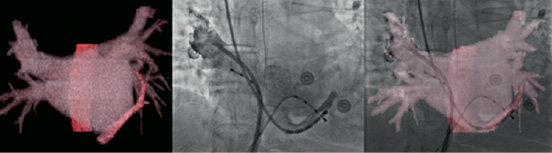
Figure 9. Imaging and registration of the left atrium: Left, CT model of CS and oesophagus. Middle, Fluoroscopic image with the oesophageal temperature probe, contrast filled right superior pulmonary vein, and CS. Right, Registered view of the CT and fluoroscopic images. Accurate overlapping of the CS catheter is seen.
Navigation
In the context of 3D enhanced fluoroscopic images, several technologies that physically navigate the catheters are being proposed, using magnetic or electromechanical guidance. Each of these approaches promises enhanced safety through precise navigation and control, broader access to interventional procedures to a larger population of physicians, reduced average procedure time and improved productivity in the cath lab. The Niobe® magnetic navigation system from Stereotaxis (Saint Louis, MO, USA) is a nice illustration of successful integration within the AXIOM Artis cath lab of Siemens (Malvern, PA, USA). Magnetic systems offer safe and efficient navigation, but they occupy significant space around the patient’s thorax, limiting access, and available projections with imaging system. Furthermore, they require magnetic shielding of room, flat panel technology (II cannot tolerate magnetic field) and dedicated devices incorporating a magnet near their tip. Some restrictions on patient selection may include patients with implanted pacemakers. Electro-mechanical guidance is a simpler alternative to control catheter deflection, via fine cables in the catheter walls. The advantages of such a system are the rapid, direct and localised control, close to hand control. Higher force can be applied for procedures like septal punctures. Moreover, the control device can be embedded in a very small container, and such a system is compatible with any room, and any device, at low cost17.
Conclusion
In the era of drug-eluting stents and emerging non-invasive diagnosis modalities, one can reasonably question why and how angiography will evolve in the coming years. We have reviewed the technological revolution and how we foresee that it will enable safer, more ambitious therapeutic percutaneous procedures. These improvements will ultimately permit treatment of a broader variety of patients with procedures that are less invasive than their surgical counterparts.
As non-invasive diagnostic techniques advance for vascular disease assessment, we believe that the cath lab will increasingly become an interventional theatre and a place where images obtained from different modalities will be integrated to tack advantage of what they have to offer over real-time fluoroscopy. From this perspective, the cath labs of the future will benefit from an open platform, with flexible and standardised interfaces and displays, able to accommodate any new emerging imaging modality. Such progress will allow new minimally invasive procedures, not limited to coronary artery diseases, but also in the domain of structural heart and heart failure.

