Introduction
In 2009, the new editorial team of the European Heart Journal established the European Society of Cardiology (ESC) Journal Family (Figure 1) with manuscript transfer1. In order to work together efficiently, the editors of all specialty journals were invited to become senior consulting editors of the European Heart Journal.
Indeed, with the growing number of submitted manuscripts (a total of 3,700 in 2012) and the ever higher impact factor of the European Heart Journal currently peaking at 14.1, the acceptance rate has dropped to 10%2,3. This is unfortunate as many manuscripts are of interest for more specialised cardiologists. Since the introduction of manuscript transfer (Figure 2), 1,053 manuscripts have been transferred to subspecialty journals, 334 of them to EuroIntervention. In fact, EuroIntervention has received 612 submissions with a respectable impact factor of 3.173 making transfers attractive for authors. However, impact is not the only aspect of a journal’s influence; its impact as assessed by downloads is equally important4,5. Of note, in the European Heart Journal, 3.1 million documents are downloaded every year; in EuroIntervention, 130,000 PDFs per year.

Figure 1. The ESC Journal Family.
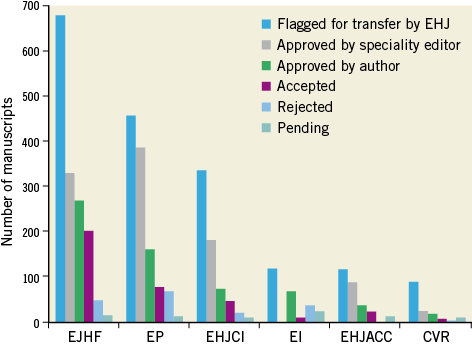
Figure 2. Manuscript transfer from 2009 to the present within the ESC Journal Family.
Manuscripts may be transferred without review (and are then considered on a regular basis by the subspecialty journal) or with reviews provided by the European Heart Journal. The latter allows authors –provided the editor of EuroIntervention agrees– to resubmit a revision based on these reviews. This is faster and more convenient than a submission to another journal and has led to an intense and most enjoyable collaboration between the two journals.
In addition, with the regular symposium at EuroPCR we have further strengthened this partnership. In this review, the last EuroIntervention/European Heart Journal symposium held in Paris on May 23, 2013, is summarised.
Percutaneous coronary interventions (PCI) in stable CAD
USE OF A NEW GENERATION OF DES
Drug-eluting stents (DES) perform better than bare metal stents (BMS), but the question remains whether new-generation DES are superior to first-generation DES. The SCAAR-STENT Registry assessed the risk of restenosis, stent thrombosis (ST) and death for BMS (n=64,631) in first-generation DES (n=19,202) and novel DES (n=10,551) in an all-comers registry over two years6. The risk of restenosis was lowest in new DES (HR: 0.62) as compared to the first-generation DES (HR: 0.29) and BMS. The risk for ST was also lower (HR: 0.57) than with first-generation DES (HR: 0.38) or BMS as were death rates (HR: 0.77 vs. first-generation DES and HR: 0.55 vs. BMS).
However, an all-comers study design does not ensure that all consecutive patients are evaluated. As shown by de Boer et al, only half of the population are enrolled due to refused informed consent and/or participation or exclusion criteria7. Accordingly, baseline characteristics of participants and non-participants differ and short-term mortality is higher in non-participants.
PCI FOR CHRONIC TOTAL CORONARY OCCLUSION
The five-year clinical outcome of the PRISON 2 study randomising 100 patients each with chronic occlusions to sirolimus-eluting stents (SES) or BMS revealed lower target lesion revascularisation (TLR; 12% vs. 30%; p=0.001), lower target vessel revascularisation (TVR; 17% vs. 34%; p=0.009) and major adverse cardiovascular events (MACE; 12% vs. 36%; p=0.001) with SES8 with no differences in death and MI. Thus, in patients with total occlusions the known favourable outcome of DES is sustained up to five years.
REVASCULARISATION OF LEFT MAIN AND 3-VESSEL DISEASE
Kappetein et al presented the three-year follow-up results of the SYNTAX trial comparing coronary artery bypass grafting (CABG; n=897) and PCI using paclitaxel-eluting DES (n=903) in patients with left main and/or 3-vessel disease9. At three years, MACE defined as death, stroke, myocardial infarction (MI) and repeat revascularisation occurred in 20.2% of CABG and in 28% of PCI patients (p<0.001) (Figure 3). The repeat revascularisation rate was 10.7% and 19.7% (p<0.001) and the MI rate 3.6% and 7.1%, respectively (p=0.002), and hence higher in patients treated with PCI. In contrast, the safety endpoint of death/MI/stroke was not different (12% vs.14.1%). Overall, at three years, MACE was significantly higher in PCI- as compared to CABG-treated patients. The authors concluded that in patients with less complex CAD (i.e., low to intermediate SYNTAX scores) PCI appears an acceptable revascularisation strategy.
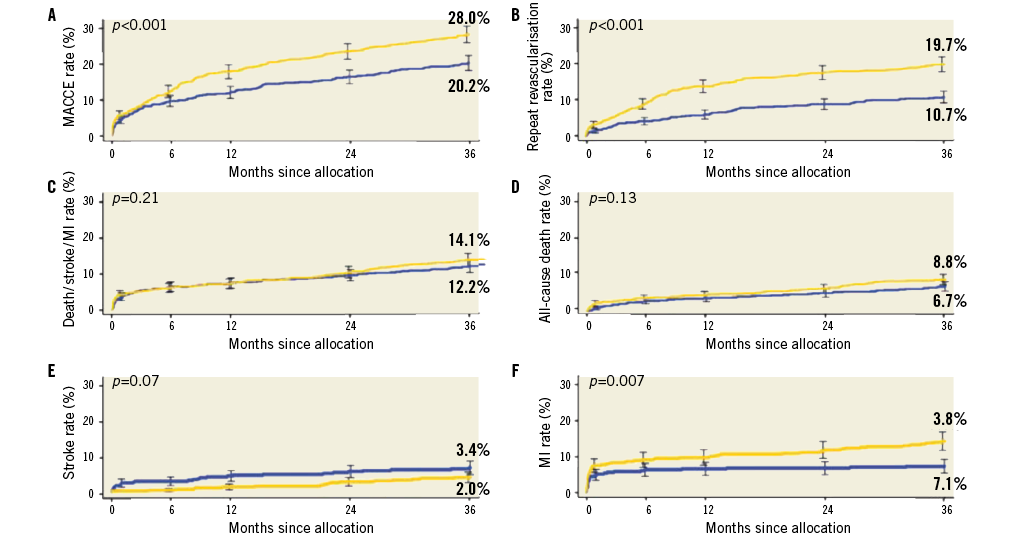
Figure 3. Rates of clinical outcomes among randomised treatment groups. Time-to-event curves in patients treated with coronary artery bypass grafting (blue line) or percutaneous coronary intervention (yellow line) for the composite of major adverse cardiac and cerebrovascular events (A), repeat revascularisation (B), death/stroke/myocardial infarction (C), all-cause death (D), stroke (E), and myocardial infarction (F) to three years. P-values from log-rank test9.
Farooq et al reported as well from the SYNTAX trial that the correlates of four-year mortality were multifactorial10. The strongest correlates with mortality were lack of aspirin or post-procedural antiplatelet treatment and peripheral vascular disease. Farooq has also proposed a SYNTAX tool for CABG11.
FFR-GUIDED PCI AND LONG-TERM OUTCOME
Li et al reported the seven-year outcome of FFR-guided versus angiography-guided PCI that was assessed in a large retrospective study of 7,358 consecutive patients referred to the Mayo Clinic12.The outcome of PCI only (n=6,268) was compared to FFR-guided (n=1,090) with FFR <0.80 as an index of a significant stenosis during a follow-up of 50.9 months. MACE at seven years were 57% in the PCI-only and 50% in the FFR-guided group (p=0.016). FFR-guided deferred PCI (n=721) was associated with a reduced risk of MI (HR: 0.46; 95% CI: 0.26-0.82; p=0.008). This suggests that, in contemporary practice, an FFR-guided strategy is associated with a favourable long-term outcome that extends the favourable outcome of previous short-term FFR-guided trials.
Noninvasive computer tomography-based FFR (CT-FFR), recently reviewed by Serruys et al13, is an emerging noninvasive alternative to FFR that may mature into a comprehensive CAD imaging technique prior to revascularisation.
PERIPROCEDURAL MI
The frequency and causes of periprocedural MI defined as CK-MB >3x times upper limit of normal was reported among 23,604 patients of 11 prospective PCI studies by Park et al14. The frequency of periprocedural MI was 7.1%. In half of the cases, side branch occlusion was the cause. After adjustment for baseline variables, patients with periprocedural MI had a higher risk of mortality (HR: 1.20). This underscores the current guideline recommendation that non-ischaemic lesions as assessed with FFR should not be treated with PCI.
ISCHAEMIA-GUIDED REVASCULARISATION
Revascularisation with prior myocardial perfusion scintigraphy is a recommended approach in symptomatic patients with stable angina. It is seen that large retrospective studies favoured revascularisation over medical treatment in patients with moderate to large ischaemic areas. However, no distinction was made between the extent of reversible ischaemia and scars. Hachamovitch et al assessed the impact of ischaemia and scar on the benefits of revascularisation in an observational study (n=13,969) with a follow-up of over seven years using adenosine or exercise stress SPECT myocardial perfusion (MPS)15. The percent of myocardial ischaemia and scar was calculated using a five-point/segment MPS scoring. Frequency of early revascularisation was assessed at 12 to 18 months (n=1,226; 9%) and all-cause death at seven years (n=3,893; 29%). In patients without prior MI and more than 10% ischaemic myocardium, early revascularisation was associated with survival benefit compared to medical treatment. There was no survival benefit of revascularisation in patients with prior MI. In patients without prior CAD, prior revascularisation and prior MI, lack of inducible ischaemia was associated with better survival in patients on medical treatment.
CT-CORONARY ANGIOGRAPHY (CTCA)-GUIDED REVASCULARISATION VS. MEDICAL TREATMENT
As reported by Ko et al, ischaemia assessed by adenosine stress CT myocardial perfusion imaging (CTP) is moderately accurate in identifying perfusion defects as compared with invasive FFR16. However, preliminary, combined CT angiography and CT perfusion imaging may develop into a viable technique, particularly as the radiation exposure diminishes with more modern equipment.
Earlier coronary angiography studies have demonstrated that high-risk CAD, defined as left main and/or 3-vessel disease, is associated with better survival after coronary revascularisation compared to medical treatment in stable angina. Noninvasive CTCA is an alternative that allows the identification of high-risk CAD. Min et al reported the results of the CONFIRM Registry that assessed all-cause mortality associated with revascularisation versus medical treatment in 15,223 patients without prior CAD who all underwent CTCA17. They categorised patients as high risk versus non-high risk for CAD. Death occurred in 1.2% (n=185) during a follow-up of 2.1 years. Using a propensity score, revascularisation (2.2% mortality; n=1,103) was associated with a survival benefit for patients with high-risk CAD (HR: 0.38) as compared to medical treatment (1.1% mortality; n=14,120). There was no difference in survival after revascularisation in patients with non-high-risk CAD. These results are hypothesis-generating and should lead to future RCTs.
News on acute coronary syndromes (ACS)
THE NEW STEMI GUIDELINES
The update of the STEMI guidelines of the European Society of Cardiology18 emphasised the use of ECG within 10 minutes and atypical presentations such as left bundle block, pacemaker rhythm or ST-segment elevation on aVR leads. A more refined algorithm was produced confirming the boundary of the 120-minute delay from symptom onset to vessel opening by primary PCI, but added quality parameters (specifically, shorter delays) under certain circumstances. Further, the concept of a pharmaco-invasive approach in patients receiving fibrinolysis was reinforced with the recommendation of an immediate transfer to PCI centres after administration of the fibrinolytic. In patients with cardiac arrest, it is recommended to perform cardiac catheterisation and eventually PCI and to use therapeutic hypothermia following resuscitation. As antithrombotic agents, the new P2Y12 antagonists (i.e., ticagrelor and prasugrel) as well as bivalirudin are now recognised as gold standards.
Radial access and manual thrombectomy were upgraded to a standard option in STEMI and drug-eluting stents in patients without contraindication to dual antiplatelet therapy. Finally, early discharge in low-risk patients, mandatory left ventricle function assessment and ischaemia-driven revascularisation of non-culprit lesions in STEMI patients with multivessel disease were also recommended.
The third Universal Definition of Acute Myocardial Infarction appeared simultaneously19. In spontaneously occurring MI and after revascularisation, cardiac troponin was endorsed as the biomarker of choice for myocardial necrosis. Type 1 MI remained as the infarction secondary to plaque rupture and subsequent thrombosis, whereas type 2 MI was defined as secondary to an ischaemic imbalance.
RISK FACTORS
In spite of pro-inflammatory, pro-oxidant and pro-thrombotic stimuli operating in type II diabetes, diabetics suffering from a first MI exhibit substantially more severe coronary atherosclerosis, better collateral development and predominantly calcific plaques with less lipid quadrants and a smaller lipid core than non-diabetics as assessed by OCT20 (Figure 4). Another paradox exists in obese patients21. In the Swedish Coronary Angiography and Angioplasty Registry involving 64,436 patients undergoing coronary angiography due to ACS, the relation between BMI and mortality was U-shaped, with the nadir among overweight or obese patients and underweight and normal-weight patients having the highest risk. In addition, a new trigger to develop an ACS or cardiac arrest has been described22. The occurrence of the great East Japan earthquake disaster was associated with a rapid increase followed by a sharp decline in major cardiac events probably induced by extreme mental and physical stress.
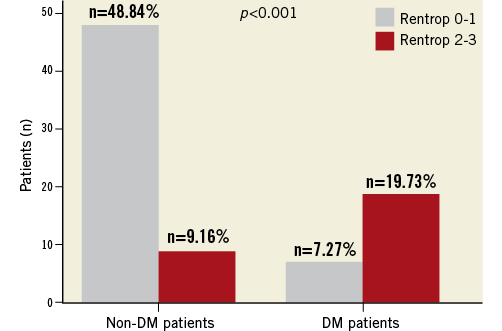
Figure 4. The rate of Rentrop 0-1 and Rentrop 2-3 in patients with and without diabetes mellitus. Good collaterals were more frequent in diabetics than in non-diabetic patients (p<0.001)20.
Discharge heart rate was a strong predictor of mortality up to four years after primary PCI in 1,453 STEMI patients23. Furthermore, major in-hospital bleeding was associated with a higher mortality beyond three years in a cohort of 32,895 NSTEMIs24.
Regarding logistics of STEMI treatment across Europe, the Stent for Live Initiative reported major increases in the numbers of pPCI performed from 2008-2010 together with improvements in STEMI mortality rates in countries involved in the Initiative25.
WHICH STENTS FOR PRIMARY PCI?
Primary PCI for the treatment of STEMI with BMS has become an established procedure, but DES may further reduce in-stent restenosis and TLR, which may be offset by a higher incidence of late and very late ST. Kalesan et al performed a meta-analysis of 15 RCTs including 7,867 patients comparing BMS with DES in patients with STEMI26. The overall risk of definitive ST with adverse outcomes was similar for BMS and DES, but the incidence of ST was lower (RR: 0.80) during the first year, but higher (RR: 2.10) thereafter for DES. TVR was much less frequent (RR: 0.51) for DES.
Boden et al reported the outcome of the MISSION RCT27. They evaluated five-year survival and MACE of primary PCI with BMS compared with DES in 200 patients treated for STEMI. There was no significant difference in survival with BMS and DES (92.8% vs. 94.3%) nor in re–MI (13.7% vs. 10.6%). TVF and ST rates were also similar.
The COMPARE trial evaluated the effectiveness at two years of everolimus-eluting stents (EES; n=434) compared to paclitaxel-eluting stents (PES; n=429) in patients with acute MI with all-cause death, re-MI and TVR as primary endpoints28. At two years, EES was superior to PES (9.2% vs. 16.1%; p<0.001), most striking in STEMI compared to non-STEMI. Furthermore, ST rates were lower with EES than PES (RR: 0.30; p=0.005). Thus, the safety and clinical efficacy of a second-generation DES is superior to PES in patients with acute MI as had also been reported by the COMFORTABLE investigators29. This suggests, as well, that very late ST associated with first-generation DES may be less common with new-generation DES.
OTHER TECHNICAL ASPECTS
Two large meta-analyses of STEMI treatment via the transradial approach suggested a superiority as compared to the transfemoral access30,31. The impact of thrombus aspiration in the real world32 confirmed a reduced mortality with this technique. However, conflicting results of a randomised trial33 and the fact that only a minority of interventional cardiologists are currently using this technique indicate the need for a definite trial34. Another controversial aspect of pPCI is the treatment of the culprit lesion only versus complete revascularisation in STEMI patients with multivessel disease35,36. Overall, current findings support a delayed treatment of non-culprit lesions over ad hoc treatment. In this regard, the CULPRIT trial aims to address this issue within an RCT37.
REPERFUSION INJURY AS A THERAPEUTIC TARGET
Exenatide provided myocardial salvage when administered at the time of pPCI in a randomised trial38. In another study, erythrocyte-rich thrombi with more inflammatory cells and high thrombus burden appeared to be associated with impaired myocardial reperfusion in STEMI patients39. Post-conditioning has also been the subject of several reports40-42. Although a randomised trial revealed no benefit of this technique in pPCI41, a meta-analysis of 10 randomised trials of STEMIs treated by pPCI (n=560) suggested that post-conditioning might confer protection in terms of myocardial enzyme levels and left ventricle ejection fraction particularly in young, male patients, and when direct stenting was used42.
Structural heart disease
A decade ago transcatheter aortic valve implantation (TAVI) was launched as a less invasive alternative to surgical aortic valve replacement for patients with severe aortic valve stenosis at high operative risk43. Recently, the updated ESC/EACTS guidelines on valvular heart disease formulated a strong recommendation for TAVI in patients at (very) high operative mortality risk, if the decision was made within a multidisciplinary Heart Team44.
THE VARC CRITERIA
Both the European Heart Journal and EuroIntervention reported on the outcome data as well as the technological progress of TAVI. Of note, both Journals jointly published the second Valve Academic Research Consortium (VARC) document, an integrated effort of academic research organisations, tertiary care institutions and medical authorities to provide uniform definitions on TAVI-related clinical endpoints45,46. Today we see that the VARC definitions have been adopted in ongoing registries and randomised trials. The importance of uniformity in reporting outcome data cannot be underestimated and will facilitate future study comparisons. VARC-2 provided updated definitions and emphasised novel TAVI-related concepts such as refined risk stratification and relevant risk variables (i.e., porcelain aorta, frailty, hostile chest), comprehensive echocardiographic recommendations and quality of life assessments. An important TAVI-related complication is stroke and VARC-2 underscores the importance of proper neurological assessment both before and after TAVI and proposes the need for involvement of trained neurologists.
REGISTRY DATA
The multicentre Italian CoreValve Registry reported three-year follow-up data in 181 high-risk octogenarians. All-cause and cardiovascular mortality was 34.5% and 12.5%, respectively47. Mortality past 30 days post-TAVI was predominantly related to non-cardiovascular causes, notably respiratory diseases. This excess in non-cardiovascular mortality indicates that better patient selection may improve long-term TAVI outcome. Of note, at three years there were no signs of bioprosthesis degeneration as illustrated by stable transprosthetic gradients and valve areas.
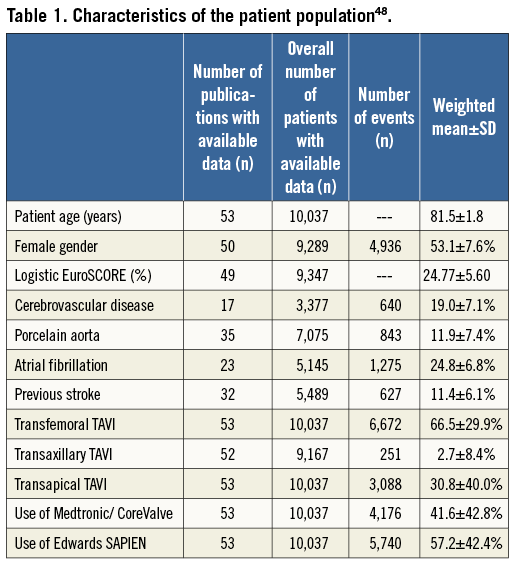
EuroIntervention focused in on the issue of TAVI-related stroke. A meta-analysis including published data on 10,037 patients from 53 studies assessed the risk of stroke after TAVI48 (Table 1). The weighted mean 30-day major stroke incidence was 2.9%. The 30-day mortality in stroke patients of 26% tripled the overall 30-day mortality. The stroke incidence was similar to another weighted meta-analysis and seemed unrelated to either device or access site49. The Bern group also reported TAVI-related stroke, including its frequency, timing and impact on outcome. Stroke incidence in 389 consecutive TAVI patients was 3.6%50. The majority occurred within 24 to 48 hours following TAVI suggesting that a periprocedural thromboembolic phenomenon and mechanical dislodgement of debris due to the inherent instrumentation within the ascending aorta and aortic root are involved. In line with this interpretation, multiple manipulations in the aortic root (>1 attempt to implant the bioprosthesis and balloon post-dilatation) were associated with a higher stroke risk. The 30-day mortality of TAVI patients suffering a stroke was approaching 50%.
Apart from stroke, most TAVI procedures are associated with subclinical brain lesions as can be documented by magnetic resonance imaging51. Embolic deflection and embolic filter devices seem appealing to reduce stroke risk and brain damage after TAVI. EuroIntervention reported on the first-in-man use of the Claret CE Pro™ cerebral protection device (Claret Medical, Inc., Santa Rosa, CA, USA) during TAVI54. Through a right radial or brachial arterial access, a filter is deployed in the brachiocephalic trunk and the left common carotid artery. Technical success, defined as deployment of the two filters, was achieved in 60% and 87% of the first and second-generation devices, respectively. Furthermore, macroscopic debris was captured in the filters in more than half of the cases. It remains to be proven whether clinically relevant neurological events can be prevented using a filter-based embolic protection device. Interestingly, apart from capturing macroscopically visible debris in three quarters of TAVI cases using the Claret CE Pro™, the debris consisted of fragments originating from the native aortic valve leaflets and the aortic wall but also contained thrombotic material53. This suggests that such devices may help to reduce TAVI-associated stroke.
The up-and-coming field – renal nerve ablation
RESISTANT HYPERTENSION
Hypertension remains the major cardiovascular risk factor despite the availability of safe and effective antihypertensive drugs. It is important to note that the percentage of patients with blood pressure at target values is low54. Patients who cannot be controlled by lifestyle modification and three or more antihypertensive drugs (including a diuretic) are defined as having resistant hypertension (RHT)54. Evidence is growing that patients with RHT are at increased risk for cardiovascular events. The Reduction of Atherothrombosis for Continued Health registry55 documented that patients with RHT and atherothrombosis had a higher risk of death, myocardial infarction or stroke (HR: 1.11-1.26, p=0.017 and 0.0008) within four years. There is thus a need for novel treatment strategies in this population.
CATHETER-BASED RENAL DENERVATION (RDN)
RDN interrupts renal sympathetic nerves and in turn reduces renal and total body norepinephrine spillover56 (Figure 5). An expert panel of the European Society of Cardiology and the European Association of Percutaneous Cardiovascular Interventions published a consensus document on patient selection, efficacy, safety, limitations and potential new indications of renal denervation57. It is recommended that patients meet the following criteria before RDN is considered: office SBP ≥160 mmHg (≥150 mmHg diabetes type 2), intake of ≥3 antihypertensive drugs in adequate dosage and combination (including a diuretic), lifestyle modification, exclusion of secondary hypertension, exclusion of pseudo-resistance using 24-hour BP (average BP >130 mmHg or mean daytime BP >135 mmHg), preserved renal function (GFR ≥45 ml/min/1.73 m2) and suitable renal anatomy (no polar or accessory arteries, no renal artery stenosis, no prior stenting).
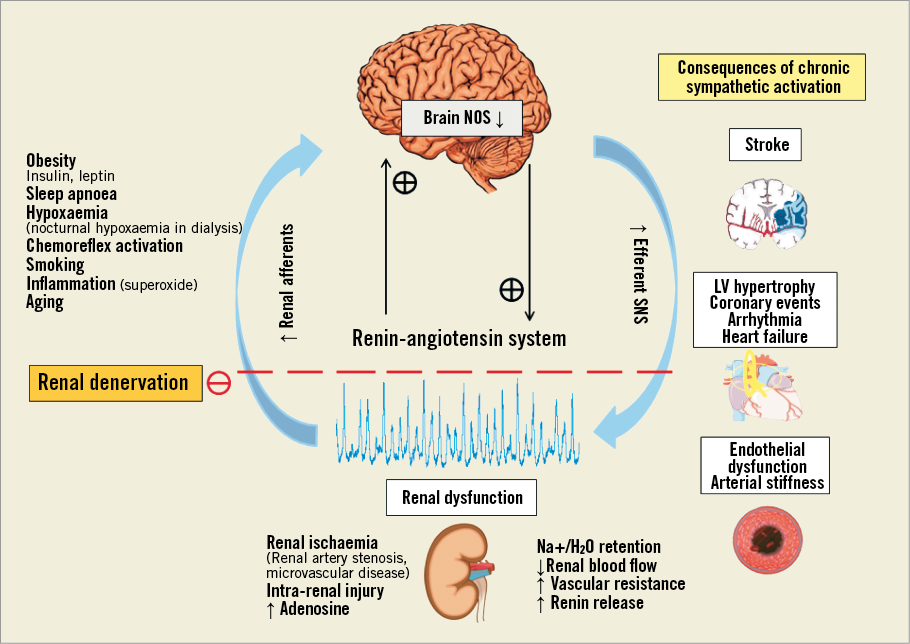
Figure 5. Mechanisms of sympathetic activation in renal disease and mechanism of activation of renal denervation72.
In patients with resistant hypertension, RDN using different devices consistently reduces office systolic and diastolic BP. However, only limited information on the impact of RDN on 24-hour BP is available58,59. A study of 346 uncontrolled hypertensives separated patients according to daytime BP into those with true resistant and pseudo-resistant hypertension60. It was found that RDN reduced office BP in all patients, while only in patients with true treatment resistance 24-hour SBP, DBP, maximum and minimum SBP were decreased. However, only a minor change in blood pressure or no blood pressure change at all was achieved in certain patients. To avoid invasive treatment by RDN in non-responders, identification of predictors is essential. RDN was equally effective in reducing BP in different subgroups of patients according to the patients’ baseline characteristics. Only office SBP at baseline has been identified as an independent predictor of BP response59-61.
LOCAL RENOVASCULAR EFFECTS OF RDN
Although radiofrequency energy can result in transient local de-endothelialisation, acute cellular swelling, connective tissue coagulation and thrombus formation in animals62, less was known about vascular injury following RDN in humans. A recent prospective study evaluated 32 arteries pre- and post-procedure by OCT; vasospasm, oedema (notches), dissection and thrombus formation were measured63. After RDN, nearly all vessels (96%) showed endothelial-intimal oedema, in 67% thrombus formation was prevalent and vasospams were observed in 42%. Authors reported one arterial dissection and two endothelial and intimal disruptions after RDN. Although controlled data are lacking, these observations suggest that antiplatelet therapy after RDN might be useful57.
TECHNICAL ASPECTS OF RDN
Numerous new catheter systems and treatment modalities are under development and currently six devices have received CE mark57. The products include new radiofrequency catheters, but also catheter-based ultrasound systems, cryoablation techniques, radiation, local drug delivery or even external application of ultrasound energy64-66. Before their use can be recommended, they will each have to prove their safety and efficacy in large cohorts with long-term follow-up67.
FUTURE INDICATIONS OF RDN
Evidence is growing that the benefit of RDN may not be restricted to BP lowering, since several cardiovascular diseases are characterised by an excessive central sympathetic drive. Indeed, chronic activation of the sympathetic nervous system has been associated with the metabolic syndrome68,69, heart failure70, arrhythmias71 and chronic kidney disease72. Promising pilot studies in these conditions have already been conducted or are ongoing.
Acknowledgements
Work of the authors has been supported by the Swiss National Research Foundation (to T.F.L. Nr. 3100-068118.02/1), the Foundation for Cardiovascular Research - Zurich Heart House (to T.F.L.).
Conflict of interest statement
T.F. Lüscher has received honoraria and research grants from Medtronic (Tolochenaz, Switzerland) and St. Jude (Minneapolis, MN, USA). M. Sabate has received speaker fees and consulting fees from Medtronic, Abbott, Biotronik and Boston Scientific. F. Mahfoud has received honoraria and research grants from Medtronic, SJM, Recor, Cordis, Deutsche Hochdruckliga, Deutsche Gesellschaft für Kardiologie, Behringer Ingelheim, Berlin Chemie, Novartis. The other authors have no conflicts of interest to declare.

