Abstract
Background: Pulmonary regurgitation may result in progressive right ventricle dilation and failure, arrhythmias and death. The implantation of a pulmonic valve at an appropriate age may restore the right ventricular function and may result in improved symptoms.
Aim: To evaluate the percutaneous Cribier-Edwards Aortic valve when deployed in the pulmonic position.
Methods and results: Acute animal testing was performed on a total of 3 pigs and 3 sheep (35-40 kg). They were catheterised through the jugular and femoral vein. The native pulmonic valve was measure by ICE and angiography. The valve was implanted using standard stent deployment techniques through a 22F sheath positioned in either the femoral or jugular vein. In three sheep the valve was small for the native valve; therefore it was implanted in the branch pulmonary artery. In the three pigs, implantation over the native valve was successful and the valve had mild angiographic regurgitation with a catheter through it. The chronic animal model consisted of two protocols.
Protocol one: Valve implantation into native pulmonary valve in 8 sheep (35-40 kg). In five, the valve was implanted through the jugular vein over a wire into the native valve without incidents and completed 20 weeks survival. In two sheep, the valve migrated acutely due to distensibility of the native pulmonary artery. One sheep had a schedule survival of 10 weeks with a paravalvular leak.
Protocol two: Valve implantation into a pulmonary conduit in 4 sheep (40-50 kg). For this, a conduit was surgically implanted (Edwards Prima Plus) between the right ventricle and pulmonary artery and the native pulmonary valve of the animals was destroyed, then a few weeks later, the percutaneous valve was implanted via the jugular vein. Three animals survived the implantation to 20 weeks. One death occurred during implantation not related to the valve. Angiography showed trivial to mild regurgitation.
Conclusions: The percutaneous implanted Edwards pulmonic valve functions well under pulmonary pressure and can be delivered from femoral or jugular vein. Acute and early chronic (20 weeks) animal studies are encouraging. Non-diseased native valve, especially in sheep is problematic for the chronic animal model. Pre-surgical implantation of a conduit into the pulmonary artery provides an ideal deployment zone for a percutaneous valve.
Introduction
Significant pulmonary valve regurgitation results in progressive right ventricle dilation that may lead to the risk of development of ventricular arrhythmias, right ventricle dysfunction and sudden death1,2. The occurrence of pulmonary regurgitation and/or obstruction is not uncommon after surgery for congenital heart defects, including tetralogy of Fallot, pulmonary atresia and any other surgical procedure requiring reconstruction of the right ventricle outflow tract1-4. Even when a valved conduit or a bioprosthetic valve has been used for this purpose, progressive pulmonary regurgitation and/or stenosis of such conduits or valves (homografts, Contegra [bovine jugular vein valve], porcine valves) can occur. Surgical pulmonary valve implantation at an appropriate age may restore right ventricular function and improve the symptoms, however cardiopulmonary bypass and ventriculotomy needed for such operations may further impair the right ventricular function3,4. Therefore timing and indications for resurrection of a competent pulmonary valve are controversial issues5,6.
In October 2000, Bonhoeffer and his colleagues reported the first animal experience of a percutaneously implanted pulmonary valve using a bovine jugular vein valve sutured inside an intravascular stent7 and, subsequently, the procedure has been reported in 59 patients8.
In December 2002, Cribier and his colleagues reported on the first human application of another percutaneous heart valve (PHV) in the aortic position9. This valve was designed initially for application only in the aortic position and the early clinical experience with this PHV in the aortic position is ongoing10.
In this paper, we report on our initial animal experience with this PHV (Cribier- Edwards valve) in the pulmonic position.
Methods
The percutaneous heart valve: The Cribier-EdwardsTM percutaneous heart valve (Edwards Lifesciences, Irvine, CA) is made of three equine pericardial leaflets sewn inside a 14 mm long stainless steel stent (Figure 1).

Figure 1. The Cribier-Edwards PHV. For comparison, on the right is the US quarter coin.
The valve is currently available in two sizes: 23 and 26 mm. The valve is mounted on a 23 or 26 mm x 3 cm ZMED-II balloon (NuMED Inc., Hopkinton, NY) catheter and delivered via a 22 or 24 Fr 25 cm long sheath (Edwards Lifesciences, Irvine, CA).
In vitro testing: This percutaneous heart valve has been previously tested under in vitro setting and demonstrated to be functional under aortic and venous pressures with durability of over 5 years. Most recently, the valve has been deployed with the aid of a specifically designed catheter (Flex catheter) that has the ability to steer the valve stent/balloon assembly inside the right ventricle to the outflow tract (Figure 2).
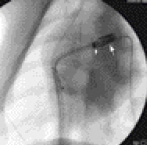
Figure 2. Cine fluoroscopy image in the LAO projection during passage of the Flex catheter (small arrow) in the right ventricle outflow tract. Note, the stent/valve (long arrow) is in front of the Flex catheter and is in the pulmonic valve position.
Acute animal model: A total of 6 animals (3 pigs and 3 sheep) weighing 35-40 kg underwent an attempt of percutaneous implantation of the PHV. The native pulmonary valves of these animals were evaluated using intracardiac echocardiography (ICE) and the valve annulus was measured. Angiography was also performed in the right ventricular outflow tract to assess the pulmonic valve position and diameter. Then a 22 Fr sheath was inserted via the right femoral or right jugular vein cut-down. A 0.035” extra stiff exchange length guide wire was positioned in either branch of the pulmonary artery and, under fluoroscopic guidance, the PHV was implanted at the site of the native pulmonary valve using standard stent deployment techniques. After the deployment, the PHV was evaluated with angiography and ICE. Following the implantation the animals were euthanized and the PHV was explanted for inspection.
Chronic animal model: This protocol consisted of 2 different groups. In one group, the PHV was implanted inside a pulmonary conduit in 4 sheep weighing 40-50 kg. The conduit, Prima Plus (Edwards Lifesciences, Irvine, CA) was surgically constructed between the right ventricle and the main pulmonary artery (Figure 3).
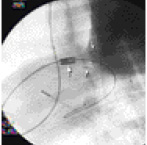
Figure 3. Cine fluoroscopy image in the lateral projection during passage of the stent/valve (large arrow) from the right internal jugular vein to the site of the Prima Plus (small arrows) constructed between the right ventricle and the pulmonary artery. The clips (small arrows) denote the position of the coronary ostia of the conduit that were ligated. The leaflets of the native pulmonary valve of the animal were destroyed during the surgical procedure so that the only competent valve was of the Prima Plus. In these animals the approach for deployment of the PHV inside the Prima Plus was via the right jugular vein cut-down. In another group the PHV was implanted over the native pulmonary valve of 8 sheep weighing 35-40 kg. In 5 animals the approach was using the jugular vein and in the other 3 it was using the femoral vein. In both groups the PHV implantation procedure was similar to that described above. All animals were observed for a maximum period of 20 weeks. Animals were receiving 100 mg aspirin in their diet daily.
Results
Acute animal model: In the 3 sheep where the PHV was implanted at the site of the native pulmonary valve, by ICE and angiography, we measured the native valve to be about 19 mm. Therefore, we expected that a 23 mm valve would be appropriate, however, the valve migrated distally and all three valves were finally placed in either branch of the pulmonary artery. Tracking the valve/stent assembly from either the jugular or femoral approach was not a problem. In the 3 pigs, the valve was successfully implanted over the native pulmonic valve. Angiographic evaluation after the implantation demonstrated mild regurgitation that could have been due to the presence of the catheter through the valve at the time of angiography.
Chronic animal models: The PHV was implanted in 4 sheep with the surgically constructed conduit. Three animals survived the implantation and completed the 20-week evaluation period. The one animal death was procedure related due to implant error. In the remaining three sheep, immediate angiography revealed trivial to mild pulmonary regurgitation (Figure 4) and this could be explained by the presence of the catheter across the valve.
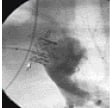
Figure 4. Cine angiography in the lateral projection immediately after implantation of the valve (arrow) in one of the sheep. This angiogram reveals competent valve with no regurgitation.
At the 20-week follow-up, one valve was competent with minimal calcium deposits (Figures 5 & 6).
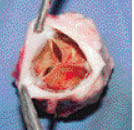
Figure 5. A specimen from one of the sheep after 20 weeks. The valve is viewed from above and demonstrates good leaflets with no evidence of significant calcium deposits.
In one animal, its native valve (supposedly destroyed during surgery) was found to be still functioning and thus there was insufficient backflow pressure to close the PHV. In another animal, the PHV did not overlap the Prima Plus valve that was still functioning and thus there was insufficient backflow pressure to close the PHV. In all three sheep, the valve was securely placed inside the Prima Plus with no embolization.
The other chronic study is still ongoing in France, where valves were implanted into the native valves of eight sheep. One of the sheep was sacrificed at 10 weeks and demonstrated good angiographic results. The results of the study will be published separately.
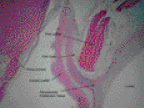
Figure 6. Histological section in the valve from figure 5 demonstrating the base of the PHV attached by proliferative spindle cell tissue to the Prima Plus conduit.
Discussion
This initial animal experience demonstrates that the Cribier-Edwards PHV can be percutaneously implanted in the pulmonary position in adult animals having pulmonary valve annulus diameter similar to that in adult humans. This is important since there is an increasing number of patients who survive surgical procedures on the right ventricular outflow tract who may develop pulmonary regurgitation and/or stenosis with a resultant right ventricle dysfunction11,12. Many of these patients have tetralogy of Fallot, truncus arteriosus or pulmonary atresia repaired using a RV-PA conduit or homograft1-4.
The Cribier-Edwards PHV is available in sizes 23 and 26 mm in diameter, which is larger than the Bonhoeffer valve that is available up to 22 mm. This is relevant since many patients have larger conduits or outflow tracts that will require a larger valve to be implanted. A PHV selected just similar (or smaller) to the diameter of the conduit will expose the patient to the risk of displacement and embolization of the valve stent as occurred in some of the first animals in our series using the 23 mm PHV. Perhaps, in those animals, even significant over-sizing of the valve may have resulted in more stable position (use of the 26 mm valve for an outflow that measures about 19-20 mm) than the use of the smaller valve. In these animals, their native outflow tract proved to be very distensible and implantation of even a valve that was 3-4 mm larger than the measured outflow tract resulted in distal migration of the valve. Furthermore, the implantation over a surgically constructed RV-PA conduit resulted in a more stable position for the PHV, suggesting that implantation in patients with RV-PA conduits or homografts can offer a better anatomic substrate to perform the PHV implantation.
Bonhoeffer reported on a technique to percutaneously “downsize” the Right Ventricular Outflow Tract (RVOT) using a specially designed self-expandable stent allowing the uneventful implantation of PHV in large RVOT13. Although this technique needs refinement, it is possible to speculate that a previously stented RVOT conduit also could be an excellent substrate for implantation of the PHV. Stenting the RVOT is a standard procedure in many cardiac centers dealing with such patients14. Therefore, we believe that the use of the Cribier-Edwards valve will be either: 1) a procedure done where the main problem is regurgitation and the valve stent can be deployed anywhere in the conduit or 2) if there is an associated narrowing of the conduit, placement of a regular stent initially will set the stage for placement of the PHV.
The preferred vascular access for this technique is the femoral vein. However, many patients with congenital heart defects undergo multiple surgical and catheterisation procedures and their femoral veins will not be patent. As we demonstrated in our animal trial, this valve/stent also can be safely delivered from the jugular vein, rendering this technique available to most patients.
Bovine pericardium has been used to construct leaflets for valvular bioprostheses and has been used clinically for more than 20 years. It still remains one of the preferred materials15. Our results also demonstrate that the equine pericardial leaflets explanted at 20 weeks after implantation did not demonstrate significant degeneration or calcium deposits, suggesting a behavior similar to the available bioprosthetic materials used in valves.
The implantation of a PHV can be a significant contribution to therapeutic interventions since it offers an alternative treatment modality to avoid the deleterious effects of cardiopulmonary bypass and ventriculotomy in patients who usually undergo multiple prior interventions. The benefits obtained after the replacement of the pulmonary valve in patients with free pulmonary regurgitation and right ventricle dysfunction can be attenuated due to the effects of cardiopulmonary bypass and ventriculotomy. The less invasive percutaneous pulmonary valve implantation can result in higher rates of right ventricular function recovery than surgical valve replacement. At least it would avoid surgical morbidity and it could offer a shorter recovery period and hospital stay. Percutaneous pulmonary valve replacement has already been reported to improve right ventricular parameters and clinical status similar to patients after surgical replacement8.
The valve was implanted on December 13th, 2005 in a 16-year old patient with significant narrowing of his right ventricle - pulmonary artery (RV-PA) conduit. After the conduit was stented using a P3110 (Cordis, A Johnson & Johnson Company) stent mounted over a 20 mm balloon, the 23 mm Cribier-Edwards valve was deployed inside the stent. This resulted in improvement of the right ventricle pressure from 80% to 50% systemic with no pulmonary regurgitation. The next step will be to evaluate the valve in selected groups of patients.
Acknowledgment
The authors wish to thank Mr. Larry Wood, Mr. Laksen Sirimanne, Mr. David Taylor, Mr. Rajesh Khanna, Mr. Assaf Bash, Mr. Chris Chia, Ms. Bella Felsen, Ms. Luanne Bailey, Ms. Holly Whitin from Edwards Lifesciences for their help and support and the staff of IMM Research in Paris for their help in the animal trial.

