Summary
Stem cell transplantation could be an attractive adjunctive therapy to the conventional primary percutaneous coronary intervention (PCI) in the treatment of acute myocardial infarction (AMI) to salvage ischaemic myocardial tissue and preserve long term myocardial function. Indeed, feasibility of myocardial salvage by endothelial progenitor cells (EPC) or pluripotent stem cells has been shown previously under various experimental and clinical conditions and appears to have entered the arena of interventional cardiology. Transplantation of autologous mesenchymal or haematopoietic stem cells and EPCs permit long term myocardial delivery of various therapeutic growth factors at the site of ischaemic insult (with angiotrophic, anti-apoptotic and chemotactic properties) or alternatively aid myocardial function by trans-differentiation into endothelial cells or cardiomyocytes. These cells assist in the generation of new vessels with an increase in myocardial perfusion and promote myocardial salvage following acute myocardial ischaemia leading to long term preservation of local and global contractile function. Several multicentre clinical trials have shown promising results of stem cell therapy in the treatment of acute and chronic myocardial infarction on global ejection fraction, as well as local myocardial contractile function and perfusion1-4.
The widespread utilisation of this novel biotechnology in coronary catheterisation laboratories is limited by the availability and costs associated with the use of a GMP-level cell processing facility for expansion of allogeneic or autologous cells to achieve a therapeutic dose. In contrast, real time isolation of cells from adipose tissue using the Celution™ system enables the introduction of cell therapy to a conventional coronary catheterisation laboratory without specific infrastructural or logistic requirements. This device can be operated by a single technician, generating autologous adipose derived regenerative cells (ADRCs) within about one hour following tissue collection.
This enables application of the cell therapy directly following the primary coronary intervention after the AMI. Using the Celution™ cell separation system, adipose tissue obtained by standard, manual liposuction is processed into a single cell suspension devoid of adipocytes which contains pluripotent stem cells, EPCs and other stromal cells. Various large and small animal acute myocardial infarction (AMI) and chronic myocardial ischaemia (CMI) studies have clearly demonstrated the feasibility, safety and efficacy of intracoronary and intramyocardial transplantation of ADRC preparations. These animal studies have shown preservation of post-AMI left ventricular ejection fraction, reduction of cardiac remodeling and an increase in myocardial perfusion, without evidence of side-effects or arrhythmias. First clinical evaluations using the Celution™-isolated adipose derived stem cell transplantation via intracoronary and intramyocardial delivery in patients with AMI and CMI are imminent (APOLLO and PRECISE trial). Enrolment is anticipated to start as early as December 2006.
Introduction
Cell therapy in cardiology may constitute a novel interventional technique to stimulate myocardial salvage and neoangiogenesis following myocardial infarction which has generated a great deal of attention with intensive research in the last few years. The potential of improving myocardial contractile function following AMI beyond restoration of perfusion has created a lot of interest and expectations. Nevertheless, practical challenges need to be resolved in order to provide a commercially viable therapy from a company’s perspective, as well as an affordable and widely amenable form of therapy from a socio-economic perspective.
Device description
The Celution™ System (Figure 1) is a CE-marked bedside device that allows the fully automated isolation of autologous adipose-derived regenerative cells (ADRCs) at the point of care within approximately one hour.

Figure 1. The Celution™ system: Bedside, point of care device for the fully automated isolation of autologous adipose derived stem and regenerative cells.
The closed system, due to its single-use disposables, renders the cost-intensive GMP cell culturing facilities unnecessary and allows the clinician to generate a high yield, single cell suspension of stem and regenerative cells at the bedside for immediate application, which can be directly appended within the same procedural setting. This approach allows the treatment of patients admitted with AMI with cellular therapy that have a short therapeutic window where there would be no room for time consuming cell culture expansion to achieve a therapeutic dose. Additionally, this approach also improves cost effectiveness of treatment of patients with CMI by obviating the need for second hospitalisation for delayed treatment.
The process is started with the harvest of a few hundred millilitres of adipose tissue by standard manual liposuction through a stab incision under local anaesthesia using a Toomey syringe and cannula. This procedure can be performed during the primary coronary intervention or at the bedside. During the fully automated isolation using the Celution™ System, the adipose tissue is enzymatically digested into a single cell suspension, and washed. Subsequently, all lipid-laden adipocytes and debris is separated from the therapeutic ADRCs, which eventually contains a combination of adult mesenchymal-like stem cells, endothelial progenitor cells and other adipose tissue stromal cells.5-8 The cell isolate can then be applied immediately to the patient (Figure 2) either by percutaneous intracoronary or intramyocardial injection.
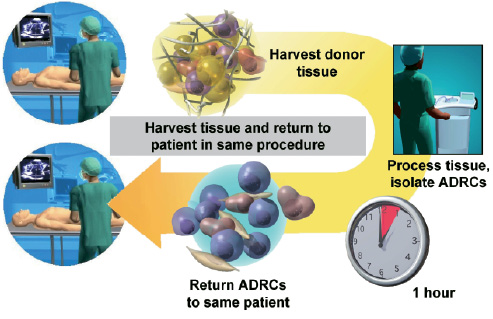
Figure 2. Adipose tissue harvest, ADRC isolation and application in the same procedural setting using the Celution™ system.
The modular design of the Celution™ system makes further refinement (by cell selection, or additives) of the ADRC population possible for future tailoring to a wide variety of cardiovascular diseases, since the ADRC transplant has been shown to, not only augment myocardial salvage, but also promote contractile function in chronic myocardial ischaemia models.
Preclinical data
Adipose tissue as a source of multi-potent stem and regenerative cells5,6 is easily accessible without special surgical staff or infrastructure requirements, and can be obtained in adequate quantities by manual syringe liposuction. The cell population isolated by the ambulant Celution™ system is void of adipocytes and is comparable, but not identical to bone marrow derived mesenchymal cells7,8. In addition, the adipose tissue derived cell preparation consists of different cell types, including multi-potent adult stem cells, endothelial progenitor cells, differentiated endothelial cells, smooth muscle cells, and pericytes, that normally can be found in the adipose microvasculature. Application of these autologous ADRCs in small and large animal models of AMI has shown consistent therapeutic benefits due to three main mechanisms of action: 1) induction of neoangiogenesis, 2) preservation of myocardial wall thickness and 3) improvement of contractile function. More importantly, both intracoronary and intramyocardial delivery has been safe and well tolerated, showing no indication of cell-related morbidity or arrhythmias in small and large animal AMI models.
In a randomised study in rats, autologous ADRCs (treated) or saline (control) were delivered into the left ventricular chamber (an approximation of intracoronary delivery), following a one hour occlusion of the LAD. After twelve weeks, cell-treated rats showed a significant improvement in global ejection fraction on echocardiographic analysis over control animals (76% versus 68%, p<0.05). In addition, the capillary density was significantly improved by 40% in the myocardial border area as compared to saline controls (p=0.007).9
In a pig AMI model, ADRC transplantation 48 hours after infarct induction resulted in a preservation of global left ventricular function as compared to the groups receiving the carrier solution only. Left ventricular ejection fraction in animals receiving ADRC therapy (n=13) was improved by 11% as compared to the control group (p<0.01) at six month follow-up.10
These robust improvements of post-myocardial infarction contractile function (as measured by LVEF) were reproduced in two additional porcine studies using cultured11 (n=21, 4 week follow up) and freshly12 isolated ADRCs (n=16, 8 week follow up; manuscripts submitted). Moreover, 99mTc-SestaMIBI scan demonstrated an absolute reduction in myocardial perfusion defects by 6% and 9% over control animals, using respectively cultured cells (p=0.01) or immediately isolated and applied cells (p=0.037). This reduction in perfusion defect could be linked to a retention of the myocardial wall thickness and an increase in the vessel density in the border zone of the infarcted region.13
Clinical experience using ADRCs
The Celution™ system is currently in clinical use, in an investigator-initiated breast reconstruction trial in Japan. Women, who have undergone partial mastectomy due to cancer resection, undergo treatment with autologous ADRCs and fat tissue to reconstruct the tissue defect. Preliminary results on volume retention as well as other outcomes are anticipated in 2007. Several clinical case reports have been published to date with the use of autologous ADRCs. One case study reported the successful use of autologous ADRCs in a child to stimulate bone repair after two autografts failed to repair a critical calvarial defect.14 In other case reports, autologous ADRCs were injected into the rectal mucosa of several patients to successfully stimulate local healing of chronic fistulas resulting from Crohn’s disease,15,16 or they were used in plastic surgery to promote retention of transplanted autologous fat tissue during surgical soft tissue augmentation17.
Cardiac clinical trials using the Celution™ System
Cytori Therapeutics plans to initiate several clinical trials in Europe in acute myocardial infarction and chronic myocardial ischaemia in 2006/2007 using the CE marked Celution™ cell isolation system. The APOLLO Trial is planned as a randomised, placebo-controlled, double-blind, dose escalation safety and feasibility study for the intracoronary treatment of AMI using ADRCs and will enrol up to 48 patients. The PRECISE Trial is planned as a randomised, placebo-controlled, double-blind, dose escalation safety and feasibility study in up to 36 patients with chronic ischaemia, who are not eligible for coronary artery bypass surgery or PCI. These patients will receive intramyocardial injections of ADRCs using the NOGA® mapping and delivery system.

