Summary
Background: A 69-year-old male patient with severe asymptomatic carotid artery stenosis was treated percutaneously with implant of two self-expandable stents in the right carotid overlapped to each other by 5 mm. The 15-month follow-up colour-Doppler ultrasound (CDU) revealed a severe stenosis in the target vessel and an image suggesting migration of the distal stent.
Investigations: Physical examination, laboratory test, CDU, carotid angiography, quantitative carotid angiography (QCA), brain computed tomography (CT).
Diagnosis: Migration of the distal stent associated with severe stenosis on the unsupported arterial segment.
Management: Carotid artery angiography, QCA, antithrombotic therapy, carotid artery angioplasty and stenting (CAS).
How should I treat?
Presentation of the case
A 69-year-old male patient was referred to our hospital to treat a severe asymptomatic stenosis of the right internal carotid artery (ICA) in March 2000. He had a clinical history of dyslipidaemia, obesity, tobacco use and asthma. In 1995 he suffered from an acute myocardial infarction (AMI) complicated by cardiogenic shock. Due to unstable angina a coronary angiogram was performed in January 2000 that revealed occlusion of the circumflex and right coronary arteries. We decided for the maintenance of the medical treatment due to unsuitable coronary anatomy for either percutaneous or surgical treatment. In the same exam a severe (95%) ulcerated stenosis was diagnosed at the origin of a very tortuous right ICA (Figure 1A); a moderate (50%) stenosis of the left ICA; an infrarenal abdominal aorta aneurysm; and a moderate stenosis of left iliac artery.
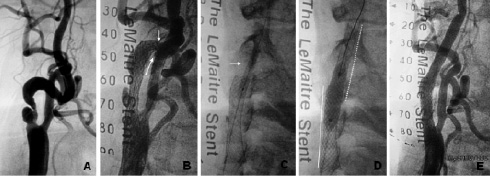
Figure 1. (A) Severe ulcerated stenosis of right ICA. Note the tortuosity of proximal ICA segment; (B) Self-expandable stent was placed in the proximal portion of ICA. It was observed a severe stenosis distally to stent (white arrows). (C) Dilatation from stent distal edge with balloon 3.5 x 20 mm (white arrow). (D) Post-dilatation in the stent overlapping zone with 7.0 x 20 mm balloon (full line - first implanted stent, dotted line - second implanted stent). (E) Final angiographic result.
These findings were in accordance with a physical examination of abdominal and neck murmur. Echocardiogram confirmed left ventricular dysfunction with an ejection fraction of 30%. CDU revealed a severe (95%) ulcerated lesion in the right ICA constituted mainly by a fibrotic component, and a moderate stenosis of left ICA. Laboratory tests did not reveal any changes in haematological and biochemical parameters, except by a cholesterol level of 222 mg/dl. A chest X-ray showed an increased cardiac area. Brain CT did not detect any signs of ischaemic lesions. The patient was under medical treatment with a daily dosage of: ticlopidine 500 mg, aspirin 100 mg, simvastatin 20 mg, diltiazem 180 mg, oxitropium bromide 100 microgram, nitroglycerin 250 mg, enalapril 20 mg.
After discussing the case with vascular surgeons and the patient, it was decided to treat the right ICA stenosis by CAS. Under local anaesthesia with lidocaine, an 8 Fr introducer was inserted into right femoral artery. Unfractionated heparin (100 IU/kg) was administered to achieve an activated clotting time (ACT) > 250 sec. Right common carotid artery was cannulated with an 8 Fr multi-purpose guiding catheter. A 0.014-inch BMW guidewire (Guidant Corp., Santa Clara, CA, USA) was positioned in the distal ICA segment. Atropine 1 mg was administered to avoid severe bradycardia. Lesion predilatation was carried out with a 3.5 x 20 mm Maxxum balloon (Boston Scientific Corporation, Maple Grove, MN, USA). A 7.0 x 30 mm monorail Carotid Wallstent (Boston Scientific Ireland Ltd, Ireland) was positioned at the bifurcation level leaving its distal edge proximally to the tortuous arterial segment. After stenting, a severe stenosis was observed distally to the stent distal edge (Figure 1B). It was speculated that it could be caused by accentuation of vessel tortuosity, arterial spasm or arterial dissection. Nitroglycerin was administrated at a total dosage of 800mcg without any result. Multiple dilatations were performed with a 3.5 x 20 mm Maxxum balloon in the stent distal edge (Figure 1C). After those manoeuvres, it remained a severe stenosis with angiographic aspect of arterial dissection. It was decided to implant another 7.0 x 30 mm Carotid Wallstent (Boston Scientific Ireland Ltd, Ireland) from the distal edge of first stent, overlapping with it by approximately 5 mm. After stenting, a postdilatation with 7.0 x 20 mm Marshal balloon (Boston Scientific, Natick, MA, USA) at low pressure (6 atm) was performed at the level of the overlapping zone (Figure 1D). A final angiogram revealed an acceptable result with clear straightening of the proximal ICA segment (Figure 1E). The control cerebral angiogram revealed no changes from the basal. The patient was discharged home the following morning after CDU confirmed good CAS result.
The CDU performed at one, three and nine months and the one year follow-up revealed no abnormalities. CDU at 15-month follow-up showed an image that suggested migration of the distal stent associated with a fibrotic stenosis on the uncovered arterial segment between both stents, with peak systolic velocity flow of 2.20 m/sec. A new carotid angiogram was performed in July 2001 which confirmed migration of the distal stent resulting in dissociation of the two stents leaving approximately 8 mm of uncovered arterial segment between them (Figure 2).
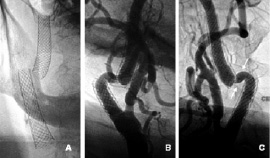
Figure 2. (A) Fluoroscopy image of two dissociated stents. There is no evidence of stent fracture; (B) Left anterior oblique view showing stent dissociation and a severe stenosis in the proximal edge of the distal stent; (C) Right anterior oblique view showing the uncovered arterial segment between stents (white arrows).
This segment had a marked tortuosity and the appearance of a severe stenosis in the proximal edge of distal stent. The left ICA presented a progression of lesion severity to 75% stenosis.
The case was discussed with vascular surgeons, clinical cardiologist and the patient family about the feasibility as well as the risks of: 1) maintenance of medical treatment; 2) a new CAS procedure; and 3) vascular surgery.
How could I treat?
The Invited Experts’ opinion
The case, even if unusual, has to be to be considered with the schematic approach we use in the daily practice. It is a case of CAS restenosis, located between two stented segments into a severely tortuous ICA.
This lesion can be classified as type I (focal <10 mm end-stent lesions)1 restenosis and could be treated with a POBA2. Unfortunately the lesion is fibrotic and located in a tortuous segment, a plain balloon dilation angioplasty will probably results into a suboptimal result. For this reason, I would consider a stent-in-stent procedure so as to achieve the best possible lumen area, despite vessel tortuosity.
Predilation could be useful to reduce friction of device positioning. Concerning stent selection, ideally a hybrid cell design stent3 would be the choice, but this a nitinol stent. Personally, even if more rigid, I would adopt a same alloy stent of the ones used in the previous procedure. Stents overlap, proximal and distal, should be robust so as to achieve an appropriate imbrication of the new stent, this must also be secured with stent post-dilation.
This technique will require some neuroprotection. In this case, the ICA is severely tortuous and the first choice for neuroprotection should be a proximal protection device4. Unfortunately, in this case the stent mesh will preclude appropriate device positioning in the ECA. Thus we need to accept a distal protection system. Among the available systems on the market, I would use a bare wire based filter (Emboshield Pro, Abbott Vascular, Galway, Ireland) for the following reasons:
1. The bare wire is more trackable and can be relatively easily placed in such tortuous distal ICA.
2. After wire positioning, the operator can test the hypothesis of having the bare wire beneath some of the stent struts by advancing a PTCA balloon. If the balloon will advance up to the wire distal tip, then the possibilities of having the wire between the stent’s struts and the vessel wall are minimal. Then, the filter can be advanced on the bare wire and deployed.
3. Finally, the filter can be advanced on the bare wire and deployed. A huge advantage of this kind of filter is that, when deployed, it allows independent wire movement. This is important because we would need to advance a relatively bulky stent into a tortuous, already stented vessel, which could require wire manipulations and even a push and pull technique.
In conclusion, thanks to the growing experience with endovascular approaches to recurrent lesions and the technological advances in neuroprotection devices, we should be able to avoid the need for an operation in this case.
The surgeon’s point-of-view
The authors have provided an interesting and very unusual complication after carotid stenting of an asymptomatic high-grade stenosis of a severely angulated internal carotid artery.
Although severe angulation is considered a contra-indication for carotid stenting, I can understand the choice of a minimally invasive procedure in this patient with severe co-morbidity.
During the initial intervention the authors have chosen a relatively short stent (30 mm). Nevertheless, this Carotid Wallstent is a rigid stent which is known for straightening the internal carotid artery. The cause of the stenosis after implantation of the first stent remains unclear, although it can be expected that straightening and deflecting forces can cause such a stenosis. The choice of a second stent in this situation is intuitive and the final result is, as mentioned by the authors, acceptable. It should be acknowledged that some interventionists would have preferred an open cell design, and therefore a more flexible stent for such a complex and tortuous lesion.
From the follow-up angiography, it can be appreciated that the distal stent has migrated and that the segment in-between is stenotic.
To my knowledge there is no report of stent dislocation in the carotid area, but, this is an unusual situation with two stents in the internal carotid artery. Most likely the dislocation of the more distal stent is caused by head motion. Internal carotid artery stents can lead to alterations of cerebropetal blood flow. An MRI-study assessed three-dimensional anatomy and volumetric flow rate in the ICA in various head positions by comparing patients treated with carotid angioplasty and stenting with patients treated with carotid endarterectomy.1,2 Investigations were performed in different head positions. There was a significant increase in internal carotid artery angulation in stented patients if the head was bent forward. This angulation change did not lead to significant acute changes in cerebropetal blood flow, but it might have chronic effects not yet tested.
How should I treat?
There are several options to treat this unusual complication.
– Conservative treatment: Since this is an asymptomatic patient, there is no emergent indication for treatment. Studies on asymptomatic lesions showed that the absolute risk reduction of surgery for an asymptomatic lesion was 5% at 5 years compared to best medical treatment. Sub-analysis has shown that surgery was mainly beneficial in younger and healthier patients.
– Surgical treatment: is not an option in this case. The distal end of the second stent is surgically inaccessible for clamping.
– Interventional therapy: Through a femoral access it will be possible to pass a guidewire through both stents. A third “bridging stent” can be positioned with sufficient overlap in the first and second stent. Considering the angulation, an open cell design would be most appropriate.
Hesitating between the first and third option, the last would be my preference.
How did I treat?
Actual treatment and management of the case
Percutaneous treatment was accepted as the first therapeutic option by all parties. A new CAS procedure was carried out in July 2001. An 8 Fr sheath was inserted through the left femoral artery after local anaesthesia with lidocaine. Unfractionated heparin (100 IU/kg) was administered to achieve an ACT > 250 sec. The brachiocephalic trunk was cannulated with an 8 Fr Zuma 2 Hockey Stick guiding catheter (Medtronic, Minneapolis, MN, USA). A 0.014-inch Choice PT guidewire (Boston Scientific Scimed, Maple Grove, MN, USA) was positioned in the intracranial segment of right ICA supported by a 3 Fr intracoronary infusion catheter (Ultrafuse-X, SciMed, Maple Grove, MN, USA). The infusion catheter was advanced through both stents to confirm that the guidewire did not cross between stent struts, and left distally to the second stent. Using the infusion catheter as an exchange catheter, the Choice PT guidewire was changed by a 0.014-inch Platinum Plus guidewire by 300 cm in length (SciMed/Boston Scientific, Watertown, MA, USA) with the aim of increasing support (Figure 3A).
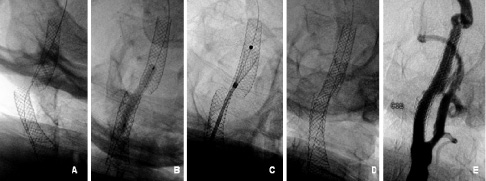
Fig. 3. (A) A stiff 0.014” guidewire in the distal ICA segment; (B) Predilatation between both stents with 4.0 x 30mm balloon; (C) Positioning and releasing a 7.0 x 30mm Carotid Wallstent; (D) Final fluoroscopy; (E) Final angiographic result.
The infusion catheter was removed, leaving the guidewire in place. Predilatation with a 4.0 x 30 mm U-Pass balloon (Cordis Europe, Roden, The Netherlands) was performed between both stents (Figure 3B). A 7.0 x 30 mm Carotid Wallstent (Boston Scientific Ireland Ltd, Ireland) was centred between the two stents and deployed (Figure 3C). Postdilatation was carried out using a 6.0 x 20 mm and 7.0 x 20 mm Submarine Balloons (Invatec Corp., Brescia, Italy) both at 6 atm to increase stent wall apposition. Completion angiography confirmed resolution of the vessel tortuosity and stenosis resulting in a widely patent ICA (Figure 3D/F). The control intracranial angiogram revealed no perfusion defects. Hemostasis was reached with Angioseal 8 Fr (St. Jude Medical, Stratford Upon Avon, United Kingdom). The patient was discharged on the following morning after an uneventful period.
Follow-up and additional procedures
The patient also submitted to a successful CAS of the left ICA under cerebral protection, in January 2002. At this same occasion, an angiographic control from the right carotid, representing the six month follow-up was performed confirming a good result of the previous treatment. The CDU follow-up performed at one, three and six month as well as three years after the last procedure confirmed a persistent and acceptable result of the bilateral carotid procedures without any aspect of restenosis or stent migration.
Discussion of diagnosis
Diagnosis of stent migration was suspected by the 15-month follow-up CDU and confirmed after carotid angiogram. Fluoroscopy acquisition showed two stents completely separated from each other (Figure 2A) with no evidence of fracture; and the angiogram acquisition showed an unsupported arterial segment between the stents (Figure 2B/C). Three hypotheses were formulated to explain these images: 1) the distal stent migrated distally from its original position; 2) the distal stent remained on its original position but its proximal edge suffered a continuous foreshortening process during time; 3) both processes happened simultaneously.
To clarify those hypotheses, image analyses were performed using the QAngio®Medis XA version 7.1 software. Two angiograms frames were used during analyses as they had precise markers for distance calibration: 1) the postdilatation frame of the first procedure (Figure 1D), with available markers of a 20 mm balloon and 2) the predilatation frame of second procedure (Figure 3B), with available markers of a 30 mm balloon. The proximal and distal stents lengths of both procedures were measured as well as, the overlapping segment length of the first procedure and the unsupported arterial segment length of the second procedure (Figure 4).
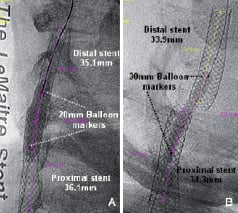
Fig. 4. (A) First CAS procedure: measurement of stents lengths. Calibration performed using the markers of a 20 mm balloon; (B) Second CAS procedure: measurement of stents lengths. Calibration performed using the markers of a 30 mm balloon.
Measured data are showed in Table 1.
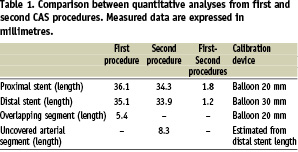
Some conclusions were reached after analysing these data:
– The proximal stent remained on its original position. Its length reduced from 36.1 to 34.3 mm, probably due to a continued process of stent diameter expansion with concomitant proximal edge foreshortening.
– The distal stent was clearly dislodged from its original position. Its length reduced from 35.1 mm to 33.9 mm, probably due to a continuous process of stent diameter expansion with concomitant proximal edge foreshortening. The total “foreshortening length” was of 1.2 mm, not explaining the total distance travelled by the distal stent, which was calculated as the sum of the overlapping zone length of the first procedure (5.4 mm) plus the uncovered arterial segment of second procedure (8.3 mm) for a total distance estimated of 13.7 mm.
The third hypotheses of stent foreshortening and true stent migration was thus confirmed. We speculated that over time, the tortuous arterial segment that was straightened continuously forced the distal stent to return to its original anatomy pushing the stent up as showed in the Figure 2C. The known characteristic of axial foreshortening of braided mesh stent1-3, mostly related to its proximal edge, was also verified in this case but it was not solely responsible for the whole observed process.
Several authors have published cases of stent embolisation or migration during coronary interventions4-11, but stent migration after the procedure is an unusual complication and mostly described in literature when related to the use of self-expandable stents in the venous system or the use of balloon-expandable stents in the arterial system2,12-21. Of course, those findings are related to the frequency of use of each device in these vessels, as in theory self-expandable stents have a lower risk of migration if compared to balloon-expandable stent, even in venous system1. Distal migration of a self-expandable stent in the arterial bed is a very rare complication of percutaneous intervention and, as far as we know, this is the first reported case in the carotid district22.
Discussion of initial CAS
The indication for the initial percutaneous procedure was based on the grade of stenosis severity and patient’s high surgical risk according to NASCET trial23 (previous unstable angina, LVEF < 35%) and others comorbidities. When analysing this procedure, it is essential to correlate the lesion characteristics and vessel anatomy to technical characteristics of available devices at the time of procedure: 1) the right ICA had a severe (95%) ulcerated lesion what in theory requires a stent with higher scaffolding proprieties; 2) the accentuated tortuosity of the proximal segment of ICA could become a problem in terms of filter devices and stents with low flexibility properties, considering that both devices could have rectified this segment and increased the procedural risk. At the time of the initial procedure, proximal cerebral protection devices and nitinol stents were not yet available on the market. With regard to performing a CAS procedure without a cerebral protection device, it was considered that the risk of manipulating the distal vessel segment could be higher than the risk of cerebral embolisation by the procedure itself. The initial strategy was to not use a cerebral protection device and to stent the lesion without touching the distal vessel’s tortuosity. In fact, after releasing the first stent, it was noticed that the stent’s distal edge was a little bit higher than expected, “touching” the tortuous vessel segment. Its mechanical action could have developed an increase in vessel tortuostiy, arterial spasm and/or vessel dissection. As the final aspect was compatible with artery dissection associate to severe narrowing of the artery, the solution was to implant a second stent overlapping the first one. Of course, when releasing a non-flexible stent in so tortuous an arterial segment, the initial anatomy drastically changed becoming a straight shape. Even changing the original vessel anatomy, the control carotid angiogram (Figure 1E) revealed an excellent final result.
Nowadays, to treat this specific case, we would have chosen a proximal cerebral protection device and a hybrid nitinol carotid stent that has a central segment of closed cells with a high coverage property as well as proximal/distal segments of opened cells with high flexibility to deal tortuous arterial segments.
Discussion of second CAS (treatment of stent migration)
The treatment of the stent migration was decided to be conducted percutaneously due to the patient beiong at high risk for surgical intervention and to the technical difficulty of the surgical approach due to the presence of a stent in a high level of ICA. On the other hand, to maintain the patient only on medical treatment would not be a proper decision as the uncertain situation of a “migrating stent” did not have a predictable evolution. The third stent worked as an anchor to both stents, increasing the radial force to fight against the vessel’s tendency to reacquire its original anatomy. Again, a CAS procedure was performed without a cerebral protection device, but this time the reason was that secure information of guidewire positioning (not crossing between the struts) had been taken. Earlier this was not available on the marked filters devices with free guidewire choice. Using the intracoronary infusion catheter, it was possible to verify the guidewire luminal position as well to change it to a stiffer one, increasing support during the procedure itself. Secure information of a fibrotic plaque component was also available, as shown by CDU, which carries a lower probability of distal embolisation.
We believe that when considering performing an overlapping between two self-expandable stents, the distal stent should have little foreshortening and good flexibility. Those characteristics are found in open cell nitinol stents.
Conclusion
A careful review of this case suggests that anatomical vessel features such as severe tortuosity may influence CAS procedural complication rates and so must be approached with dedicated materials in a “tailored CAS” concept. The evolution of interventional devices such as proximal cerebral protection devices and nitinol self-expandable stents has favoured the handling of distal vessel tortuosity. A long term follow-up is crucial for this patient in order to detect late and unsuspected complications of CAS. This case also suggests that late complications of CAS can be successfully managed using endovascular techniques.

