The universal use of drug-eluting stents has significantly reduced the problem of restenosis and changed the practice of interventional cardiology in an unprecedented way1. However, in patients at risk for aspirin / clopidogrel withdrawal, those, who require elective surgery, and those with a history of bleeding disorders, the risks and benefits of drug-eluting stents must be carefully weighed. Recent reports of late stent thrombosis2, loss of vasomotion3, hypersensitivity reactions4, and late restenosis5 have highlighted potential shortcomings of this powerful technology and rejuvenate the interest in alternative strategies which promote, rather than inhibit, repair mechanisms following stent implantation.
Rationale for titanium as stent surface material
Stent surface material importantly affects neointimal hyperplasia and thus restenosis. Gold6-8 as stent coating has unexpectedly shown an increase in late loss and need for repeat revascularisation compared with stainless steel stents. The likely mechanism for this observation was found in experimental data, documenting increased inflammatory reactions in gold coated as opposed to stainless steel stents. Similarly, patients with nickel allergy have been reported at increased risk for restenosis following stent implantation9. Nickel constitutes an important component of stainless steel, again suggesting that stent material plays an important role in the vascular repair mechanisms following stent mediated arterial injury. More recently, hypersensitivity-like reactions and eosinophilic inflammation have been reported in the RADAR project following implantation of drug-eluting stents, most likely related to the polymer coating used for drug-release4.
Titanium has superior biocompatibility compared with stainless steel, gold or cobalt-chromium10,11 such as high corrosion resistance and low tissue reaction characteristics. As nitride-oxide alloy, titanium can be easily deployed by physical vapour deposition on the surface of stainless steel stent. In-vitro examinations of titanium-nitride-oxide demonstrated diminished platelet adhesion and fibrinogen binding compared with stainless steel12. Using electron scanning microscopy we were able to show that the coating is less than 1 micron thick and consists of titanium, nitrogen and oxygen. The energy-dispersive-x-ray detection method indicated that nitrogen and oxygen are present in a ratio of 1:1. Chemical-elementary analysis confirmed the presence of NO-particles (Figure 1) on the surface of the titanium coating.
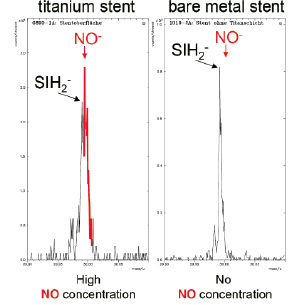
Figure 1. Typical spectrogram of a titanium-nitride-oxide coated stent (left) and an uncoated bare-metal stent (right). The titanium-coated stent shows a large NO-peak on its surface which is not present in the bare-metal stent.
These observations indicate that stent coating with titanium-nitride-oxide is associated with NO-particles on the stent surface. The release characteristics have not been studied so far, but the presence of NO on the stent surface might be beneficial with respect to intimal proliferation and platelet aggregation. Previous investigations have shown that NO prevents platelet aggregation and reduces proliferation of smooth muscle cells13. Preclinical studies with a titanium-nitride-oxide coated stent in the porcine restenosis model showed a 50% reduction of neointimal hyperplasia compared with an uncoated bare metal stent of otherwise identical design12. The antiproliferative effect was comparable to that reported by Suzuki14 for sirolimus-eluting stents in pigs. The beneficial results of a titanium-nitride-oxide coated stent were subsequently confirmed in a small scale, randomised, multicentre, clinical trial15. Mean late loss was significantly lower for titanium-nitride-oxide coated stents (0.55±0.63 mm) than uncoated, stainless steel stents (0.90±0.76 mm). Similarly, angiographic restenosis (15% vs. 33%, P=0.07) and target lesion revascularisation (7% vs. 23%, P=0.07) trended to be lower with the titanium-nitride-oxide coated stent at 6 months follow-up.
Registry experience with a titanium-nitride-oxide coated stent
In the current issue of EuroIntervention, two registries are published showing experience with the commercially available Titan™ (Hexacath, France) stent in everyday clinical practice: a nine-month-follow-up report from the Titan Pori registry, and the multi-centre Titan registry from Israel. In the Pori registry, 193 patients with 212 lesions were included at a single centre and followed for 6 months. The registry comprised approximately one third of all patients derived from routine clinical practice, with a high proportion of acute coronary syndromes (57%) and complex lesions (Type B and C: 88%). The mean reference vessel diameter (2.9±0.3 mm) and lesion length (12.9±3.0 mm) are indicative of vessels with a moderate risk of restenosis. The rate of major adverse cardiac events (MACE) at 9 months was 10.4% with a low target lesion revascularisation (TLR) of 5.2%. Notably, there was no case of stent thrombosis up to 9 months, but clopidogrel was administered for a mean duration of 8 months.
In the Israeli registry, 296 patients were included, 36% had diabetes mellitus and 81% acute coronary syndromes. The vessel characteristics revealed a high proportion of complex lesions (Type B2 or C: 61%) and long lesions (49% > 15 mm, mean lesion length: 17.5±14.8 mm). The overall rate of MACE at 6 months was 7%, consisting of TLR in 5.4% of patients, myocardial infarction in 0.7% of patients, and death in 0.7% of patients. Myocardial infarction was related to acute and subacute stent thrombosis in two patients, but clopidogrel was administered for only one month.
The two studies indicate that stent coating with titanium is associated with favourable clinical outcome. The need for repeat revascularisation was low as was the incidence of stent thrombosis. Due to the absence of a drug and polymer, there is no need for prolonged clopidogrel administration. Indeed, clopidogrel was administered for only one month in the study by Mosseri et al, similar to bare metal stents. The Titan stent platform is further characterised by a very low stent profile, high flexibility, and low strut thickness of 80 µm, and therefore provides for excellent deliverability especially in calcified, tortuous vessels out-performing current generation drug-eluting stents. Clinical studies comparing thick- with thin- strut stent designs revealed reduced restenosis and target lesion revascularisation16,17. Therefore, the combination of titanium-nitride-oxide stent coating with this thin strut construction might have allowed a reduction in restenosis. Finally, the cost for the Titan stent is only approximately half that of current drug-eluting stents. In a cost-conscious health care environment, the incremental benefit of drug-eluting stents compared with the Titan stent deserves consideration especially in light of the recent BASKET trial18, where drug-eluting stents proved only cost-effective in high-risk groups.
Future directions
While the present registry data with the Titan™ (Hexacath, France) stent are encouraging, the following limitations have to be considered. Both registries included only a moderate number of patients and lesions with a limited follow-up period of 6 to 9 months. Patient selection was at the discretion of the operator, and assessment of clinical outcome was not adjudicated. Therefore, comparison of these data with other registries of bare metal stents and drug-eluting stents may be of limited value. What is then the role of the Titan™ stent in the current era of drug-eluting stents?
First, coronary artery stenoses at low to moderate risk for restenosis, i.e. lesions in vessels with a reference vessel diameter >3.0 mm and length of <15 mm may be more cost-effectively treated with a titanium-nitride-oxide coated stent than drug-eluting stents.
Second, there is increasing concern that drug-eluting stents are associated with late stent thrombosis requiring long-term dual antiplatelet therapy. Accordingly, patients who are not candidates for long-term dual antiplatelet therapy such as those scheduled for elective surgery after the index procedure, patients at increased risk of bleeding, those requiring oral anticoagulation, and the elderly may be more appropriately treated with a titanium-nitride-oxide coated stent.
Third, in light of the favourable angiographic and ultrasonic measures of restenosis with the titanium-nitride-oxide coated stent in concert with its promising safety profile, the time might have come to compare this stent directly with drug-eluting stents in randomised, clinical trials.