Description
The SR200 MyoCath™ is a endoventricular needle-injection catheter utilizing catheter tip deflection technology and a adjustable depth, spring-loaded needle to provide multiple and precise delivery of biologic injectate to targeted myocardium.
History
MyoCath™ intellectual property was secured in 1999 and 2000. Initial prototypes were created and design was finalized in 2000. Bench testing, design control testing, biocompatibility/toxicity testing, thrombogenicity testing, in vitro testing and in vivo testing were all completed in 2001. MyoCath™ is manufactured in an ISO certified, class 100,000 controlled environment room and has been in clinical use since 2002.
Technical specifications

Indication for use
MyoCath™ is an investigational device indicated for injecting an aqueous solution or suspension, for diagnostic or therapeutic purposes, into internal tissues or organs.
Tips and tricks for delivery (use)
– Use of a steerable guidewire is not required.
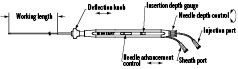
– Slight deflection of the catheter tip facilitates crossing the aortic valve when performing endoventricular injections.
– A 9F guide catheter can be used if additional back-up support is required to reach difficult areas (i.e. septal) of the left ventricle.
– During long procedures, flushing the catheter with cold saline restores torquability and back-up support.
– Using deliberate force to extend the needle and infusing the injectate slowly over several seconds helps to optimize channel creation and injectate retention in scarred areas of myocardium.
– PVCs and tactile feel will confirm presence of the needle against the myocardial wall.
– Mixing injectate with non-ionic contrast enhances visualization of injectate retention under fluoroscopy.
Preclinical experience
As part of the design validation effort, Bioheart, Inc. performed ten in vivo studies in the porcine model to assess performance and ease of use of the MyoCath™ in the hands of a number of experienced interventional cardiologists. The primary objective was to ensure the design is suitable for its intended use under simulated conditions in the animal model. Physicians rated the performance of the device using the following metrics:
– Device preparation
– Ease of access
– Ease of placement
– Needle core advancement / retraction
– Needle insertion / removal
– Device injection
– Device visualization
– Ease of removal
– Thrombogenicity
– Device integrity
– Overall performance
– Standard anticoagulation regime
– Comparison with standard practices
Overall, the physicians rated the MyoCath™ favorably and reported it performed in an acceptable manner.
Clinical experience
The MyoCath™ has been used in 25 clinical cases to-date. All clinical cases involved injections in chronically infracted left ventricles (septal, anterior and posterior locations were targeted). No adverse events related to MyoCath™ have ever been reported, and feedback captured from physicians following completion of these cases was highly positive.

