Abstract
Excimer laser coronary atherectomy (ELCA) has been an FDA-approved treatment for coronary artery disease since 1992 and is commonly used as an adjunct treatment to balloon angioplasty. While ELCA presents a good therapeutic option for lesions non-dilatable or uncrossable with balloon angioplasty, historically the technique was not able to manage heavily calcified lesions. The advent of a smaller (0.9 mm tip diameter) excimer laser coronary catheter (Point 9, Spectranetics Corporation), capable of delivering higher energy densities and repetition rates, has proven effective and safe in the treatment of complex calcified lesions1-3. However, there is still a need for a smaller laser catheter for use in small vessels in other anatomical locations, balloon-resistant lesions, and for primary crossing of CTOs. We recently developed a new, even smaller over-the-wire (OTW) laser catheter (Point 7) capable of accessing the most distal vasculature and providing an additional margin of safety in treating complex lesions. Combined with a simple guidewire steering element this device could possibly be used to traverse the proximal cap of CTOs and assist in the primary crossing of the blockage.
Introduction
History
Excimer lasers were invented in the mid-1970’s4. They produce brief, intense flashes of UV light that allow the vaporisation of shallow layers of tissue or plaque, typically a few microns in depth. After hundreds of pulses, macroscopic holes can be drilled without collateral effects to the surrounding tissue.
ELCA was introduced in the 1980’s as an alternative method of angioplasty. The Spectranetics Corporation started the development of ELCA systems in 1986 and has since obtained FDA approval for the CVX-300® laser system and several generations of laser catheters. Since its introduction, several thousand patients with symptomatic coronary and peripheral arterial disease have been treated by ELCA5-12.
Background
Nearly three decades after the first coronary angioplasty procedure performed on a patient marked a milestone in the treatment of heart disease13, complex lesions such as calcified stenoses or CTOs remain an enormous challenge for the cardiac interventionalist. Despite advances in equipment and technique these difficult lesions may be unapproachable with current percutaneous techniques and severely restrict therapeutic options.
ELCA is a commonly used adjunct treatment to balloon angioplasty and presents a therapeutic option for lesions non-dilatable or uncrossable with balloon angioplasty. However, ELCA has historically not been able to cross heavily calcified stenoses1. The new Point 9 excimer laser catheter for treatment of complex calcified plaques is a smaller catheter (0.9 mm catheter tip diameter) capable of delivering higher energy density (up to 80 mJ/mm2) and higher pulse repetition rates (80 Hz). Histological studies on animal tissue in vitro and in live porcine lesion models showed results comparable to those obtained with previous excimer laser coronary catheters14. Subsequent clinical studies showed that the new catheter is safe and effective in patients presenting complex calcified and/or balloon-resistant lesions1-3. Even though the Point 9 catheter has proven to be very successful in treating complex lesions, its size still prevents use in very small vessels and a need for a catheter capable of accessing the most distal vasculature and balloon-resistant lesions remains. Improved flexibility could provide a greater margin of safety when operating in tortuous anatomy and could facilitate the crossing of CTOs. Furthermore, smaller and more flexible catheters could provide opportunities in neurological applications.
Objective
We present here the technical design features of a new 0.7 mm diameter laser catheter (Point 7) and the results of its pre-clinical and clinical evaluations.
Methods and results
Design/specifications
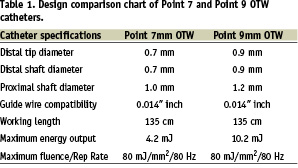
The design objective was to create a small diameter laser catheter that was compatible with 0.014 inch guidewires, 5 French guide catheters, and utilised many of the features that were successful on the Point 9 laser catheter, such as 80 mJ/mm2 fluence, 80 Hertz (Hz) laser parameters, reduced deadspace at the catheter tip, and very flexible distal end. In order to reduce the profile at the tip, a row of optical fibres was removed from the Point 9 design, resulting in a single row of 27 50-micron fibres arranged concentrically around the guidewire lumen (Figure 1).
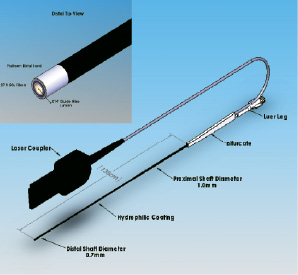
Figure 1. Point 7 laser catheter.
The guidewire lumen is constructed of thin walled PTFE tubing, 0.46 mm ID, 0.61 mm OD, with the distal section reduced to 0.39 mm ID, 0.48 mm OD, for a length of 10 cm. A 1 mm long, 0.04 mm thick platinum band surrounds the optical fibres at the tip. The band, fibres and the guidewire lumen are potted in epoxy at the catheter tip and finished with an optical polish to permit light transmission through the end of the fibres. The optical fibres are twisted (stranded) over the guidewire lumen for 30 cm to allow for equal bending stresses and low profile over the catheter distal length as described in US patent 5,415,653. An outer jacket of polyester elastomer surrounds the optical fibres and utilizes a tapered, multi-durometer configuration to allow for distal flexibility and proximal stiffness necessary for easy insertion. The distal 10 cm end of the jacket is constructed of 40D durometer polymer and is sized to fit tightly over the fibres with an outside diameter of 0.7 mm. Proximally, the jacket increases in diameter to 0.8 mm using 72D polymer for a length of 20 cm. The remaining proximal jacket length is constructed of 82D durometer material at a diameter of 1.0 mm. All jacket durometers are heat fused together to provide a smooth 135 cm long tapered shaft profile. A proprietary hydrophilic coating is applied to the distal 40 cm of the jacket. The guidewire lumen exits the catheter on the proximal end 135 cm from the tip and has a standard female luer fitting for attachment of syringes or hemostatis valves. Also at the 135 cm point, the optical fibres bifurcate from the guidewire lumen and extend approximately 3 meters within heavy walled flexible tubing to the laser coupler. Within the coupler the fibres at arranged and prepared to mate with the laser beam. The laser coupler also contains features that identify the catheter to the laser software and assist in calibration and energy delivery. Table 1 compares the design specifications of the Point 7 and Point 9 catheters.
The new Point 7 catheter features the same fluence capabilities and repetition rates as its predecessor, the Point 9. Previous catheters were only capable of delivering fluences of 30-60 mJ/mm2 at pulse repetition rates between 25-40 Hz. The lasing cycle normally used included 5 seconds of firing followed by a 10-second break during which the catheter position can be re-assessed and the saline infusion syringe can be refilled. The Point 9 and Point 7 coronary catheters are capable of delivering fluences up to 80 mJ/mm2 at repetition rates between 25 and 80 Hz and use a 10-second on and 5-second off cycle.
Regardless of catheter specifications, the preparation and technique for operation are the same. The laser system requires a 5-minute warm-up period to use. The catheter is prepared by flushing the central guidewire lumen and connecting the proximal coupler to the laser console. Calibration of the catheter is then performed, and the desired energy level is selected. The catheter is then passed over the guidewire just proximal to the lesion. Blood and contrast should be cleared from the lasing field by the saline infusion technique15 prior to each lasing train. Lasing is performed by applying gentle pressure to the catheter in order to cross the lesion under fluoroscopy while energy is emitted from the catheter tip. After the laser catheter is removed, balloon and stents are used according to standard practice.
Pre-clinical testing
All laser catheters are constructed of biocompatible materials and meet ISO 10993-1:1997 specifications for short-term blood contacting medical devices. All systems meet ISO 10555-1 specifications for bond and pull strengths and withstand a maximum infusion pressure of 300 psi (10 kg/m2).
The Point 7 catheters were evaluated for trackability and wire movement performance in a simulated heart model and compared to the Point 9 catheter design. Devices were rated for performance on a scale of 1 to 5 by 4 different operators, 5 being the best performance. Catheters were advanced over a 0.014 inch guidewire into the right coronary artery to the apex of the heart. Catheter advancement resistance was rated and documented by each operator. With the catheter in the same position, the guidewire was advanced and retracted within the catheter and the resulting movement force was rated. Results are shown in Figure 2.
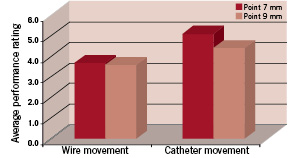
Figure 2. Catheter heart model performance: Comparison between Point 7 and Point 9 laser catheters.
The Point 7 catheter rated very similar to or higher in performance than the Point 9 catheter. Both catheters met the acceptance criteria and demonstrated the ability to access distal coronary anatomy through the simulated heart model pathway.
The ability of the catheter to ablate tissue was characterised and compared to the Point 9 catheter. Fresh porcine aorta was acquired from a local slaughterhouse and the adventicial layer was removed. The sample was immersed in saline solution in a Petri dish placed on a precision balance. The catheter tip was placed in perpendicular contact with the tissue and a known force was applied. The laser was activated and the number of pulses required to penetrate the thickness of the sample was recorded and results were calculated as tissue thickness ablated per laser pulse. A total of 10 Point 7 and 3 Point 9 catheters were tested, each producing 5 ablated holes. The results at 60 mJ/mm2, 40 Hz laser settings are shown in Figure 3.
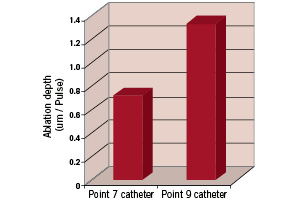
Figure 3. Ablation rate comparison between Point 7 and Point 9 laser catheters. 60 mJ/mm2, 40 Hz laser parameters.
The tissue penetration rate for the Point 7 is less than the Point 9 catheter and this can be explained by the lower fibre area in the catheter and the lower ratio of optical fibre area to catheter tip area (14% versus 20%). All catheters remained intact and functional during testing and demonstrated the ability to re-calibrate at 80 mJ/mm2 post test.
Further testing was performed to check the durability of the catheter optical faces to repeated laser pulses up against calcified tissue. Bovine leg bone was obtained from a local market, sectioned and immersed in saline. The catheter tip was placed in contact with the sample and known force was applied to the tip. 20,000 laser pulses were delivered through the catheter at 80 mJ/mm2 and 80 Hertz. The optical faces were then inspected for wear and the catheter transmission was checked by recalibrating at 80 mJ/mm2. The optical faces showed signs of normal wear at the distal tip and proximal coupler and all catheters (10) could be re-calibrated (80 mJ/mm2) post tissue ablation.
Clinical evaluation
The Point 7 catheter performance was evaluated on 17 patients presenting moderately to heavily calcified coronary and peripheral lesions with 95% to 100% diameter stenoses. The interventionalists evaluated the catheter performance on a scale from 1 (poor performance) to 3 (good performance). The results are shown in Table 2.
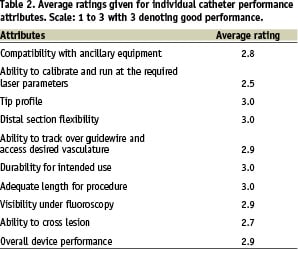
Clinicians were able to reach all target lesions with the Point 7 catheter. Laser parameters between 60 mJ/mm2/40 Hz and 80 mJ/mm2/80 Hz were used in all cases. In all but two cases the lesion could be crossed, the remaining two lesions could be crossed with a balloon after the laser treatment.
Discussion
Pre-clinical testing indicates that the new Point 7 catheter is similar in performance to the Point 9 catheter and thus safe and effective in treating high-grade, calcified lesions. FDA-approval for coronary applications was obtained in February of 2003. The extremely small diameter of the Point 7 catheter opens the door to a range of new applications such as use in stroke where the catheter needs to be advanced into the tortuous vascular anatomy of the brain, or in the treatment of lesions in small vessels found in hands or feet, or in the treatment of CTOs not crossable with a guidewire.
One CTO technique which may benefit from this smaller laser catheter is the “step-by-step” procedure used to recanalise peripheral CTOs with the laser. The step-by-step approach provides an effective means to pass through an occlusion where a mechanical guidewire has failed to pass. In this procedure, the guidewire is positioned as far into the occlusion as possible, then the laser catheter is activated and advanced to the tip of the guidewire. Once a small pilot hole is created, the guidewire is advanced further until it stalls again, then the catheter is advanced forward and the process is repeated until the lesion is fully crossed. This procedure has proven very effective in large diameter, straight peripheral vessels and many have speculated this technique may be useful in the coronary CTOs. However, previous laser catheters have been too large or too stiff, and would likely perforate the vessel wall if used in this manner without satisfactory wire guidance. The Point 7 catheter with its combination of low profile and high flexibility, may provide just enough margin of safety to allow for use of the step-by-step method in select situations outside the peripheral vasculature.
The most common reason for failure to recanalise chronically occluded coronary arteries is the inability to pass a guidewire through the occlusion into the distal lumen16. A frequent cause of failure is the inability to adequately cross the proximal cap of the occlusion with a mechanical guidewire. In this situation it would be advantageous to penetrate the “cap” safety with a laser catheter and thus allow passage with the mechanical wire. Past coronary laser catheters have been considered too large to safely ablate the cap or have not had any directional capability to allow for safely targeting the area of the cap to be penetrated. Another advantage of the Point 7 catheter’s high flexibility is the ability to use it in combination with a stiff guidewire (a steerwire) to provide directional steering capability. In this technique, very stiff-tipped mechanical guidewires, such as those used for CTOs, are used. The wire tip is shaped abruptly at the distal end, inserted into the Point 7 catheter and advanced up to the laser catheter tip. The shaped end of the guidewire deflects the tip of the Point 7 catheter approximately 20 degrees, allowing one to steer the catheter tip into position against an occlusion. This «steerwire» concept allows control over the catheter position and movement and may offer a solution for penetrating CTO caps.