Introduction
Cardiovascular disease continues to be a major health epidemic throughout the world and is the leading cause of morbidity and mortality in the Western world, with its prevalence constantly increasing in developing countries. At present, coronary artery disease, including post-infarction chronic heart failure, accounts for over 7 million deaths per year (over 2 million in Europe alone), and this number is expected to nearly double in the next 20 years1,2.
These facts clearly illustrate the need for improvement in the revascularisation and medical therapies currently available, as well as for the development of novel therapies capable of preventing or reversing negative remodelling and even regenerating failing myocardium.
In the last 10 years, repair of cardiac muscle by stem cells in patients with post-ischaemic chronic heart failure has been tested in several preclinical and phase I-II clinical studies utilising different cell types and means of delivery3-10.
Although almost all of these studies achieved promising results both in terms of safety and efficacy, few direct clinical side-by-side comparisons have been performed, and thus the quest for the ideal cell type is still ongoing. Several preclinical studies have recently been performed to compare the beneficial effects of different cell types for cellular cardiomyoplasty (Table 1). According to these preclinical studies, in the sub-acute or chronic MI setting, skeletal myoblast transplantation may be more effective than transplantation of bone marrow mononuclear cells, dermal fibroblasts, cardiac fibroblasts or adult cardiomyocytes11-14.
These results may be related to the superior degree to which myoblasts exhibit resistance to ischaemia as compared with cardiocytes. Further, myoblasts appear to have a noticeable impact on cardiac systolic function as compared with the passive effects of fibroblasts and other cell types on cardiac compliance15. To date, the feasibility of myoblasts for the treatment of CHF has been confirmed in several clinical trials in which autologous skeletal myoblasts were implanted either during open-chest surgery16-19 or via percutaneous delivery8,9,20 as a stand alone procedure (Table 2). These encouraging results have led to the design of new myoblast transplantation protocols, which are currently under investigation in ongoing clinical studies and which we hope will impart a clearer understanding of their applicability in future clinical practice.
The SEISMIC study: rationale and description
Rationale
Previous data derived from pre-clinical studies demonstrated that the implantation of autologous skeletal myoblasts may lead to replacement of non-functioning myocardial scar with functional contractile tissue and consistent improvement in global LVEF, regional wall motion and viability. Results from phase I-II clinical trials suggest skeletal myoblast implantation at the time of CABG may lead to similar effects, as do recent studies using percutaneous delivery as a stand-alone procedure. For these reasons autologous skeletal myoblast (MyoCell™) implantation using the MyoCath® endoventricular catheter delivery system is being evaluated in this randomised, controlled study to explore the feasibility these cells may provide in adding a new dimension to the interventional management of post-infarct deterioration of cardiac function in patients with congestive heart failure.
MyoCell™
MyoCell™ is a proprietary technology of Bioheart Incorporated (Sunrise, FL, USA) and is composed of autologous skeletal myoblasts expanded ex vivo from an individual patient’s skeletal striated muscle biopsy. Autologous skeletal myoblasts are isolated and subsequently expanded from approximately 10 grams of a skeletal muscle biopsy via a proprietary cell culturing process. Harvest is by trypsinisation, cell collection, and repeated washing with a commercially available transport medium to ensure removal of any residual serum from culturing. Following final re-suspension in transport medium, release testing is conducted and MyoCell™ is packaged and labelled for return shipment to the patient’s treating physician for intramyocardial implantation. MyoCell™ has been previously delivered endovascularly via specifically designed percutaneous catheter delivery systems (MyoCath®, MyoStar® and TransAccess®). In the SEISMIC study, MyoCell™ is injected into the region of akinetic myocardial scar resulting from prior infarction. Multiple injections, each containing 0.5 mL of cell suspension (25 x 106 cells/mL) and spaced approximately 1cm apart, are performed to deliver MyoCell™ to the target region. The intended dose of MyoCell™ for each patient is between 150 and 800 x 106 cells. The maximum number of injections for each implantation procedure is 32 and the maximum number of cells injected is 800 x 106.
MyoCath®
The Bioheart MyoCath® catheter (Figure 1) is a 115 cm long, 8 Fr needle injection catheter with a deflectable tip and extendable 25 G stainless steel needle. A deflection knob and needle advancement control trigger are used (Figure 2) to manoeuvre the tip, advance the needle and control the needle depth.
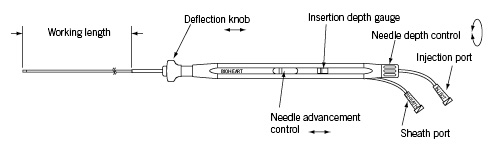
Figure 2. The Myocath™ hand piece.
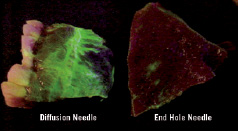
Figure 1. Cell delivery and distribution (green) performed using the new tip-closed with side holes Myocath (on the left) and the old tip with single end-hole catheter (on the right).
During the procedure, a small incision is made in the patient’s groin to provide arterial access in customary fashion. The catheter is advanced through an arterial femoral sheath retrograde across the aortic valve and into the left ventricular cavity. The injection tip of the MyoCath® is then positioned to the desired injection site of the left ventricle (via fluoroscopic guidance). The needle is advanced to a pre-set length by depressing the needle advance/retraction control, causing the needle to penetrate the target tissue to the pre-set depth. The attached syringe is then depressed to deliver the therapeutic dose (0.5 mL/per injection) to the injection site. After injection, the needle is retracted, the tip is repositioned, and another injection is made. Subsequent injections, approximately 1 cm apart, are made to all desired areas affected by prior infarct to complete the cellular cardiomyoplasty procedure.
The Bioheart MyoCath® delivery system has been used in more than 50 patients and three clinical studies worldwide without note of any serious adverse clinical events during the implantation procedure.
Study design
The SEISMIC Trial (Safety and Effects of Implanted [Autologous] Skeletal Myoblasts [MyoCell™] using an Injection Catheter) is a phase II, open-label, randomised, multicentre study designed to assess the safety and cardiovascular effects of myogenic muscle stem cells, as delivered by the MyoCath®, in congestive heart failure patients post myocardial infarction(s). Forty-six patients were targeted for enrolment at 12 study centres throughout Europe, with two-thirds of the patients randomised to the MyoCell™ treatment arm, and the other third randomised to the control arm (and receiving standard medical therapy only).
Here we report the DSMB interim analysis of the first 25 randomised patients.
Enrolment was completed in the beginning of 2007. Per protocol, all eligible patients experienced a Q-wave myocardial infarction at least 90 days prior to the surgical muscle biopsy resulting in a large area of akinesia (as confirmed by angiography and echocardiography) and residual global left ventricular ejection fraction at screening of > 20% and < 45% (as assessed by MUGA scan). Functional class was NYHA II or III without requirement, or indication for revascularisation (ruled out by angiography or dobutamine stress scintigraphy). Optimal medical therapy was to have been initiated at least 2 months prior to study entry, and all patients enrolled must have been fitted with an ICD (single-lead) at least 6 months prior to enrolment in the protocol. All randomised patients were initiated on anti-arrhythmic therapy (amiodarone) at screening, for at least one month pre- and post- procedure; both groups were followed for 6 months and evaluations were done at baseline, 1 month, 3 months and 6 months by office visits as well as by laboratory and instrument tests (ECG, echocardiography, dynamic ECG-Holter and MUGA scan at 6-month FU).
The primary safety endpoint of the study is the observed number of serious adverse events (SAE) in the treatment arm vs. control arm at 3 and 6 months, while the primary efficacy endpoint is observed improvement in LVEF (as measured by MUGA) at 3 and 6 months vs. baseline for both the treatment and control arms. Additional secondary endpoints include improvement in 6-minute walk time, NYHA classification, QOL score (Minnesota), global and regional contractility, wall thickness, coronary perfusion and changes in overall infarct size at 3 and 6 months vs. baseline. SAEs were defined by the study protocol as any adverse event deemed fatal, life-threatening, requiring unexpected hospitalisation or resulting in permanent impairment, as well as any events which the investigator deemed jeopardising toward the patient.
Preliminary safety data
Safety data was available in December 2006 on 25 of the 46 randomised patients (16 treated with Myocell™, 9 controls), with a minimum follow-up period of 30-days for all patients evaluated. At baseline, treated patients on average experienced their last MI 9.3±5 years prior to screening (range 2-21), while control patients on average were 7.1±4 years removed from their last MI (range 2-16). Ten treated patients had documented prior history of VT (63%) while 6 control patients had experienced prior VT (67%). In the treatment arm, mean LVEF was 30.0%±10.4%, with 8 patients (50%) NYHA Class III, while control arm patients entered with a mean LVEF of 32.8%±11.1%, with 3 patients (33%) NYHA Class III. On average, treated patients provided 7.9±4 grams of muscle tissue and were injected with 598±110x106 cells over 24±4 injections. All patients were treated with cells > 50% positive for CD56 staining.
Twelve non-hierarchical serious adverse events (Table 3) occurred in the treatment group in 6 of 16 patients: 1 death (due to multi organ failure), 6 VT with appropriate ICD firing (5 deemed possibly related to cell therapy and all resolved), 1 case of worsening heart failure (patient recovered), 1 case of pericarditis (patient recovered), 1 NSVT (self-resolving), 1 post-biopsy haematoma (resolved) and 1 report of herpes zoster (resolved). In the control arm, two non-hierarchical serious adverse events occurred in 2 of 9 patients: 1 non sustained VT and 1 diverticulitis, both which resolved.
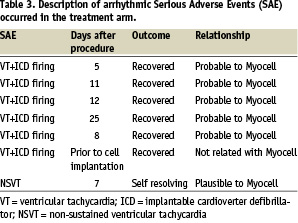
Regarding the treated patients, VTs episodes with ICD firing occurred in 4 patients (2 patients with 2 events and 2 patient with 1 event each). One patient had two separate episodes at day 5 and 11 post transplantation and, after a period of clinical recovery, progressively declined and died of multiple organ failure approximately 30 days after the index procedure. The other multi-firing ICD patient experienced 2 separate firings at 12 and 25 days post transplantation, with complete recovery thereafter and no further reports of VT. The other 2 patients experiencing 1 event each both fully recovered, with no other VTs episodes were observed. One patient experienced appropriate ICD shock 8 days post implant, while the other patient experienced appropriate ICD shock prior to the cell implantation procedure. All arrhythmic events occurred within the first month following the transplantation procedure.
Of note, 3 of the 4 treated patients experiencing 6 VT events were confirmed non-compliant with prophylactic amiodarone use per protocol, and all 4 of these patients had documented prior history of VT with ICD firing prior to entering the study.
Preliminary efficacy data
Though the limited amount of data currently available does not allow for meaningful insight into the efficacy of the MyoCell therapy, preliminary trends appear encouraging.
Six-minute walk distance data available for 3 treatment group patients and 4 control group patients demonstrate six minute walk distance improved, on average, 97±51.4 meters as compared to an average decline of 20±147.1 meters experienced by the control group patients.
NYHA Class data available for 8 treatment group patients and 6 control group patients revealed that 37.5% of the treatment group patients improved by at least one NYHA Class at 3 months following treatment as compared to 0% of the control group patients.
50% of the treatment group patients improved by at least one NYHA Class at 6 months following treatment as compared to 25% percent of the control group patients who improved.
None of the treatment group patients experienced a decline in NYHA Class at either 3 or 6 months following treatment.
LVEF as assessed by MUGA also showed a trend in improvement for the treatment group, with treated patients improving from 30.0 %±10.4% at baseline to 31.7%±21.8% at 6 months while control patients declined from 32.8%±11.1% to 31.7%±8.3% over the same time.
50% of treated patients experienced an improvement in LVEF while 57% of the control patients exhibited a reduction in LVEF.
Conclusions
Management of heart failure patients with advanced cardiomyopathy is a daunting task – patients often present with multiple medical conditions, a history of arrhythmia and little available in terms of alternative treatments to standard medical therapy. Novel therapies are needed to provide improved treatment options, and cell therapy is a promising start to this endeavour. Though complete efficacy data are not yet available and safety data are not yet fully adjudicated, these preliminary results suggest that myoblast therapy for CHF is largely safe and effective. Arrhythmic events are largely manageable with close observation and prophylactic use of ICDs and amiodarone therapy; when arrhythmic SAEs do occur, they typically appear during the first month following implantation and can largely be mitigated with appropriate medical management. Patients receiving myoblast-based cell therapy also tend to show improvement in quality of life and mechanical function over time, as evidenced in prior (completed) clinical studies and in the initial trends reported above. We look forward to receiving the final data in order to reach more definitive conclusions.
Appendix
The following investigators and institutions participated in the SEISMIC study: P.W. Serruys, Erasmus MC, Rotterdam, The Netherlands; J. Bartunek, OLV Ziekenhuis, Aalst, Belgium; V. Legrand, CHU de Liege Sart-Tilman, Liege, Belgium; W. Van Mieghem, ZOL Campus, St.Jan, Genk, Belguim: C. Nienaber, University Hospital Rostock, Germany; J. Schofer, Hamburg University Cardiovascular Center, Hamburg, Germany; C. Hehrlein, University of Freiburg, Germany; J. Waltenberger, University Hospital, Maastricht, The Netherlands; C. Macaya, Instituto Cardiovascular, Hospital Clinico San Carlos, Madrid, Spai; A. Gershlick, University of Leicester, Glenfield Hospital, Leicester, United Kingdom; N. Peters, St. Mary’s Hospital and Imperial College, London, United Kingdom; T. Siminiak, Poznan University School of Medical Sciences, Poland; P. Smits, MCRZ, Rotterdam, The Netherlands.

