Abstract
Aims: To investigate if three-dimensional (3D) based quantitative techniques are comparable to each other and to explore possible differences with respect to the reference method of 2D-QCA in the acute phase and to study whether non-invasive MSCT could potentially be applied to quantify luminal dimensions of a stented coronary segment with a novel bioabsorable drug-eluting stent made of poly-l-lactic-acid (PLLA).
Methods and results: Quantitative imaging data derived from 16 patients enrolled at our institution in a first-in-man trial (ABSORB) receiving a biodegradable stent and who were imaged with standard coronary angiography and intravascular ultrasound were compared. Shortly, after stenting the patients also underwent a MSCT procedure. Standard 2D-QCA showed significant smaller stent lengths (p<0.01). Although, the absolute measured stent diameters and areas by 2D-QCA tend to be smaller, the differences failed to be statistically different when compared to the 3D based quantitative modalities. Measurements made by non-invasive QMSCT-CA of implanted PLLA stents appeared to be comparable to the other 3D modalities without significant differences.
Conclusions: Three-dimensional based quantitative analyses showed similar results quantifying luminal dimensions as compared to 2D-QCA during an evaluation of a new bioabsorbable coronary stent design in the acute phase. Furthermore, in biodegradable stents made of PLLA, non-invasive QMSCT-CA can be used to quantify luminal dimensions.
Introduction
Coronary stenting has resolved the problem of acute recoil and late remodelling after a coronary balloon dilatation procedure1. The introduction of drug-eluting stents (DES), not so long ago, solved partially the problem of in-stent restenosis occurring in patients treated with bare metal stents1. However, the combination of an implanted metallic device into the coronary vessel wall and the elution of antiproliferative drugs from durable polymer coated stent struts for long-lasting inhibition of neointima hyperplasia (NIH) proliferation2, potentially delayed the healing process2,3. The non-endothelialised polymer coated stent struts may stay in direct contact with the bloodstream, even after a period of two years, which may possibly lead to late stent thrombosis after discontinuation of the antiplatelet therapy4,5. A possible solution to overcome this problem could be a fully bioabsorbable DES stent which will maintain the benefits of a mechanical scaffold post-balloon procedure while eluting temporarily the local pharmaceutical treatment6,7.
To investigate the effectiveness of a bioabsorbable stent made from poly-L-lactic acid (PLLA)8, coated with the proven effective NIH inhibiting drug everolimus9, a multi-modality quantitative imaging study was implemented as a sub-study of the ABSORB trial10. The ABSORB trial is the first-in-man study of the implantation of the Abbott Vascular BVS bioabsorbable stent (Bioabsorbable Vascular Solutions, Santa Clara, CA, USA).
Besides the usually applied imaging techniques to evaluate the results of new stent designs in these kind of studies (e.g., two-dimensional (2D) quantitative coronary angiography (QCA)11 and quantitative intravascular ultrasound (QCU)12, also the newly available imaging techniques of three-dimensional (3D) QCA13 and quantitative non-invasive imaging by multi-slice computed tomography coronary angiography (QMSCT-CA)14 were applied during the acute phase to evaluate their respective imaging values and future application. Metallic stents, with their inherent radiopacity hampers the luminal assessment of these devices with MSCT-CA15. PLLA does not cause MSCT-CA related image artefacts and thus could provide an improved visualisation of the lumen in BVS stented coronary segments. MSCT-CA was applied for evaluation since most patients might prefer its friendly non-invasive nature in case they participate in a longitudinal study evaluating these new stent modalities.
This study compared these imaging methodologies for the quantitative assessment of BVS stented coronary arteries.
Methods
Patients and study design
Sixteen patients (11 men and five woman, 60.9±8.6 years) were included in this sub-analysis, which was part of the multicentre ABSORB trial10. The study design has been described in detail elsewhere10, however in brief: It is a first-in-man prospective, open-labelled multicentre trial of patients with a single de novo coronary artery lesion treated with the BVS stent. The local ethical committee approved the study and each patient gave a written informed consent. Target lesions that could be covered by a 3.0 mm±12 mm or a 3.0 mm±18 mm (diameter, length) stent were selected. Locations of the BVS stent in the 16 patients were: five left anterior descending (LAD), four right coronary arteries (RCA) and seven left circumflex arteries (LCX).
BVS stent design
The laser-cut PLLA stent design consists of four major components: a high crystalline polymer backbone, a low crystalline polymer coating impregnated with everolimus and a delivery system6,10 (Figure 1).

Figure 1. This figure presents the BVS stent design. At the proximal and distal side the two golden coloured platinum markers can be appreciated.
The stent struts are 150 µm thick and at the distal and proximal edges of the stent two platinum radiopaque markers, each 135 µm, are embedded into the stent struts to facilitate accurate fluoroscopic positioning of the stent.
Coronary Arteriography including Two- and Three-dimensional (2D and 3D) QCA
Coronary arteriography was performed according to standard procedures16. Each angiogram was preceded by the injection of 1-3 mg isosorbide dinitrate. Two-dimensional QCA was performed with the CAAS II system11 (Pie Medical BV, Maastricht, The Netherlands). Three-dimensional QCA was performed with CAAS QCA-3D (CAAS 5.0, Pie Medical BV, Maastricht, The Netherlands ). This latter system uses the detected contours in the frontal and lateral images in combination with the projection data available in the DICOM format to make a reconstruction of the coronary artery segment in 3D. In this study two simultaneously acquired orthogonal views by a bi-plane system were used. The QCA measurements were performed off-line.
Intravascular ultrasound (IVUS) procedure and Quantitative IVUS (QCU)
IVUS was performed after successful implantation and additional balloon dilatation if necessary. The IVUS catheter used was a 40 MHz Atlantis™ catheter (Boston Scientific, Santa Clara, CA, USA) connected to a Galaxy™ ultrasound console (Boston Scientific, Santa Clara, CA, USA). The catheter was pulled back by a continuous speed mechanical device at 0.5 mm/s17. IVUS data were acquired after intracoronary injection of isosorbide dinitrate. All image data were digitally stored on CD-ROM and transferred later to an IVUS Picture Archiving and Communications System (IVUS-PACS).
Quantitative IVUS analysis was performed using validated QCU software (CURAD BV, Wijk bij Duurstede, The Netherlands), allowing semi-automatic detection of the intima- or stent-boundaries in longitudinal reconstructed views of the stented segment18. To overcome possible inaccuracies in quantitative analysis and problems using automatic contour detectors, which are common in non-ECG-gated acquired IVUS studies due to longitudinal catheter motion and rotation of the coronary artery around the IVUS catheter during the cardiac cycle19,20, the validated retrospective image-based pre-processing gating algorithm Intelligate® was applied21. This algorithm filters near-end-diastolic frames from a non-gated IVUS study and creates a new retrospective gated IVUS study resulting in smooth longitudinal views in which automatic contour detectors can be applied (Figure 2).
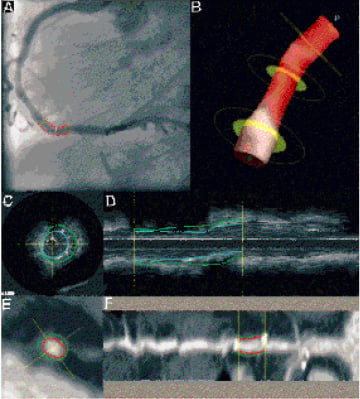
Figure 2. This figure shows examples of all applied quantitative modalities. In panel A, standard 2D quantitative coronary angiography (QCA) and in panel B, a reconstruction made by the new 3D-QCA technique. Panel C shows a cross-sectional measurement of quantitative intravascular ultrasound (QCU) from which a reconstructed longitudinal view (L-views) is presented in panel D. The blue line indicates the stent contour and the green line the external elastic membrane contour. Panel E, shows a cross-sectional measurement of the multi-slice computed tomography coronary angiography imaging (QMSCT-CA). Panel F, the reconstructed L-view, with as red contour the lumen/stent contour and the green contour the outer vessel border. The two bright spots at either side of the contour lines are the metal markers of the stent, which are causing a blooming artefact on the MSCT-CA image.
MSCT-CA procedure and Quantitative MSCT-CA (QMSCT-CA)
The MSCT-CA procedure, in a 64-slice spiral CT scanner (Sensation 64®, Siemens, Forchheim, Germany), was performed within three days after implantation of the BVS stent according to a standardised protocol22. The x-ray tube rotation time was 330 ms, resulting in a temporal resolution of 165 ms. Detector collimation was 32 x 0.6 mm with a pitch of 0.2 (table advancement was 3.8 mm per rotation). By rapid alternation of the longitudinal position of the focal spot
(Z-Sharp® Technology, Siemens, Forchheim, Germany), 64 slices could be acquired simultaneously. A tube voltage of 120 kV with tube currents between 800 and 900 mAs were applied.
Patients with a heart rate >60 BPM were given a β-blocker (up to 100 mg of metroprolol) and/or anxiolytic medication (1 mg of lorazepam) 45 min before the procedure. To enhance the coronary arteries a bolus of 100 mL of iomeprol 400 mgl/mL (Iomeron®, Bracco, Milan, Italy) was intravenously administered at a rate of 3.5-5 mL/s, depending on the size of the patient.
After the procedure, the image data was retrospective gated and reconstructed using images acquired during the middle- and late phase of diastole by software of the manufacturer (Siemens). The B30f convolution kernel was applied. All image datasets were uploaded to a local MSCT-PACS for later quantitative analysis. The coronary arteries in which the BVS stent was implanted were extracted using dedicated semi-automated MSCT-CA vessel extraction software14 (CURAD BV). This software allows the creation a new image dataset from the original MSCT-CA image data as if an intravascular imaging procedure like IVUS has been performed. After vessel extraction, quantitative analysis can be performed similar as QCU (Figure 2). Although, the contour detection is of course rather different for QMSCT-CA, the calculated parameters are similar allowing an optimal environment for comparisons between the different imaging modalities. Validation of this software has been described elsewhere14.
Measured parameters
Although the applied quantitative software packages are producing many different parameters of the coronary vessel- and stent dimensions, only a few can be used for comparison. In this study we used the mean values of the measured implanted stent lengths, -diameters and -areas to compare.
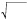
To calculate diameters from the original cross-sectional area measurements as being performed by QMSCT-CA and QCU a circular model was assumed and the following formula was applied23:
Diameter = 2x, Formula 1
To calculate areas from the original diameter measurements by 2D-QCA again a circular model was assumed and the following formula applied:
Area = πx, Formula 2
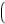
d
2

2
Statistical analysis
Quantitative data are presented as mean±standard deviation. Analysis of variance with the Bonferroni post hoc test was used to compare the mean values among groups. In these analyses a p-value of <0.05 was considered to be significant. The results were further analysed by the method proposed by Bland and Altman24, and for this study 2D QCA was selected to be the “golden standard”.
Results
All quantitative measurements for each modality were performed by a different observer, and they were blinded for the other results. In fact the measurements were also performed at different locations to prevent possible crossover bias.
Two-dimensional QCA and QCU were available in all 16 patients. Three-dimensional QCA was not assessable in five patients due to an insufficient reading of the geometry settings of the x-ray equipment. MSCT-CA could not be performed in two patients due to contrast allergies. In two of the remaining 14 patients, quantitative analysis was not possible due to poor image quality (e.g. excess of motion during the scan).
Stent lengths
Stents were identified by their metal markers on the coronary angiography images for the QCA techniques and on MSCT-CA; and by their first and last visible strut appearance in a given cross-section in the IVUS images (Table 1, Figure 2 and 3).
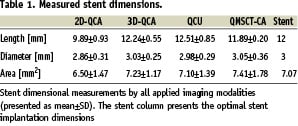
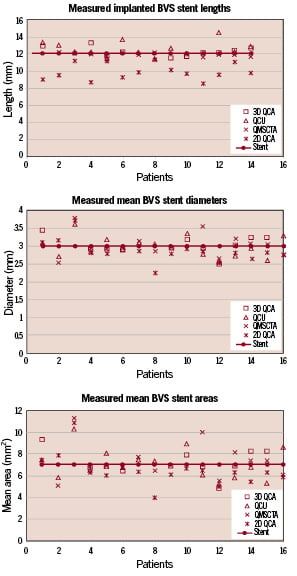
Figure 3. This graph shows all individual implanted measured stent lengths, diameters and areas as measured by the different modalities. It can be clearly appreciated that two-dimensional quantitative angiography (2D-QCA) shows significantly shorter measured lengths.
Two patients were not taken into account for comparison of the length measurements, because they received an 18 mm long stent and the 14 others were all 12 mm in length. The metallic markers resulted in a blooming artefact on the MSCT-CA images and therefore the region of interest (ROI) for QMSCT-CA was selected by clipping a small amount off the far ends of these markers (Figure 2H).
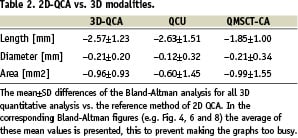
The length of the stents as determined by two-dimensional QCA was significantly shorter compared to the other modalities (p<0.001 with all modalities), with an 18% relative difference to the expected nominal stent length (e.g. 12 mm, Table 1). The Bland-Altman analysis shows the differences with the 3D based quantitative methods (Figure 4, Table 2).
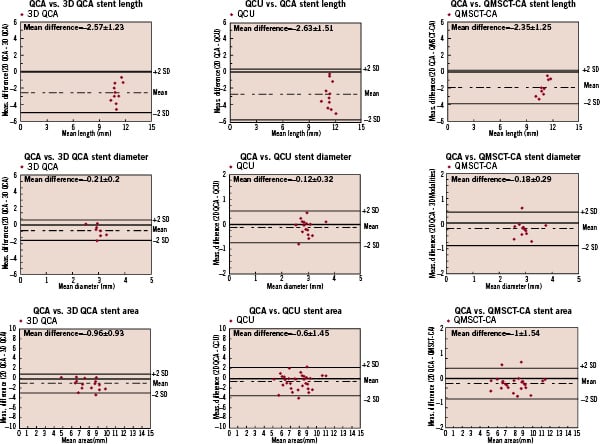
Figure 4. This figure shows all Bland-Altman analyses of the 3D image modalities were 2D QCA was used as golden standard.
Stent diameters
Comparison of the stent diameter results showed no statistical differences between the quantitative modalities (Table 1). However, 2D-QCA showed smaller absolute diameters (approximately 5%) as compared to the 3D oriented modalities and the nominal stent diameter, respectively. For the stent diameters the Bland-Altman analysis shows that in the majority of cases smaller stent diameters are measured by 2D QCA (Figure 4, Table 2).
Stent areas
Similar results were also found for the stent area measurements (Table 1). Standard 2D-QCA showed smaller absolute areas as compared to the 3D modalities, although statistically not significant, but with a relative difference of 7% as compared to the nominal stent area. For the stent areas the Bland-Altman analysis showed similar results as for the diameter measurements (Figure 4, Table 2).
Discussion
This report shows that of the applied 3D oriented quantitative coronary imaging techniques (e.g. 3D-QCA, QCU and QMSCT-CA), luminal measurements in a bioabsorbable stented coronary segment are comparable, but are showing deviation with 2D oriented QCA.
In this study three different imaging modalities were applied with four different quantitative analyses techniques to evaluate the clinical benefits of a new bioabsorbable coronary stent design during the acute phase. In the past two decades only 2D-QCA and QCU were available to study the success or failure of new interventional techniques and new pharmaceutical treatments. Recently, 3D-QCA13, an upgrade from 2D-QCA, and non-invasive MSCT-CA14 imaging became clinically available. Both having their specific advantages and disadvantages. At this point in time the imaging modalities are more complementary to each other, rather than being competitors. However, the question ought to be raised if they are comparable in simple quantitative parametric measurements as stent -length, -diameters and -areas? This study evaluated the quantitative outcome of these imaging techniques in the acute phase, directly after implantation of a new bioabsorbable stent design and the simple straightforward message is that the 3D based techniques are more in-line with each other and closer to the expected nominal stent dimensions than 2D-QCA measurements.
The measured implanted stent lengths in the 3D based-modalities were comparable but significantly different compared to 2D-QCA. The problem of foreshortening of stenoses and stent lengths by 2D-QCA has been described before11. However, 2D-QCA also showed absolute smaller stent diameters and derived areas as compared to the other modalities (Table 1)25. Although, it must be underscored that QCA diameters were converted into areas assuming a circular model (Formula 2). In this particular study, e.g. measuring a stent, the assumption of a circular model seems appropriate, although, a non-eccentric appearance after implantation of a PLLA stent is not inconceivable.
The reported smaller stent dimensions as measured by 2D-QCA as compared to QCU, are in-line with previous reports13,23. The relative concordance between the measurements obtained by the 3D-QCA, QCU and QMSCT-CA raises the question whether there is a systematic error measurement deviation by 2D-QCA, which should be corrected. A recent study to validate the 3D-QCA software also showed a 10% smaller measured diameter for a phantom introduced into a coronary artery to act as a stenosis with precisely known dimensions13.
The three selected parameters for this comparison study may seem basic, however, they are the ones most used in new stent design studies and have acted as endpoints in many multicentre trials. Furthermore, they are the most directly measured being not derived by complicated mathematical computations.
The observation made in this study is that quantitative non-invasive MSCT-CA showed results similar to the currently used reference method, e.g. QCU. Previous reports showed both under- as well as overestimation of coronary lumen dimensions as measured by QMSCT-CA when compared to the de facto reference method use of QCU14,26. However, it is difficult to compare the results of these studies with the present one, since most of them were not performed in stented segments, which immediately after stenting most likely have a circular shape (which is more easily analysable by most contouring systems). This could have enhanced the concordance between the various quantitative modalities of our study.
The metal markers render the selection of ROI with QCA and QMSCT-CA, although they resulted in a slight underestimation of the implanted stent lengths with MSCT-CA due to the blooming artefact. Leaving the metal markers out seems not to be an option in the research phase, because without them it is impossible to locate the stent using angiography and/or MSCT-CA. Furthermore, the expected bioabsorption of the struts between 2-3 years, may on the other hand make it difficult to retrieve the ROI on IVUS, if the struts are no longer visually detectable. The tiny metal markers (135 µm), may by then be embedded into the coronary vessel wall, were already not identifiable at baseline with IVUS.
Study limitations
Unfortunately, the number of patients of this first-in-man BVS stent study was limited and not every imaging modality could be applied in all patients. While showing a deviation with the 3D techniques, still 2D-QCA was applicable in all patients together with IVUS. Both MSCT-CA and 3D-QCA showed lower success rates, which should be taken into account when planning longitudinal studies. However, this is a unique patient population in which for the first time four different imaging methodologies and one new derived imaging method could be applied and used for quantitative analysis. Of course, it will be more interesting to see how the applied 3D imaging techniques perform during the long-term follow-up.
Conclusion
Three-dimensional based quantitative analyses showed similar results quantifying luminal dimensions as compared to 2D-QCA during an evaluation of a new bioabsorbable stent design in the acute phase. Furthermore, in biodegradable stents made of PLLA, non-invasive QMSCT-CA can be used to quantify luminal dimensions.

