Abstract
Percutaneous pulmonary valve implantation was successful in 83 patients over a 5 year period. During the course of our clinical experience, we encountered complications related to procedural, patient-related and device related factors. Procedural complications were predominantly related to learning curve in patient selection. Device-related factors were analysed and led to modification of the device with successful prevention in latter part of the series. Endocarditis and stent fractures were expected complications and further research into metal characteristics would be helpful to improve stent design. Research is ongoing to expand the inclusion criteria to large right ventricular outflow tracts.
Introduction
Percutaneous Pulmonary Valve Implantation (PPVI) is a novel technique used to treat pulmonary regurgitation with or without stenosis in a diverse group of patients with residual or recurrent lesions during late follow-up of adult congenital heart disease1. Over the last 5 years, 83 patients had pulmonary valve implantations with no mortality2. For any novel technique, the learning curve leads to procedural complications and early device related issues require modification of design to result in the most optimum procedural and device design factors which are then frozen to ensure safety, efficacy and reproducibility.
We describe the complications faced – and the measures used to prevent them – during the early and latter part of our experience.
Methods and results
During the course of our clinical programme of pulmonary valve implantation, we recorded all complications which may or may not have clinical or haemodynamic implications. Every complication was analysed to assess the mechanism, its clinical and haemodynamic impact, the risk of recurrence and safety mechanisms which can be used to pre-empt serious clinical events.
We categorized the complications as inherent to balloon-dilatation and stenting, procedural, device-related and unpredictable in a new technique.
Minor complications
There were 8 minor complications, which included minor dissection of the homograft (n=1), treated conservatively; detachment of the distal tip of delivery system (n=2) into the branch PA, which was snared successfully and retrieved; pulmonary haemorrhage controlled with coil embolisation of ruptured pulmonary artery branch and local bleeding at the puncture site (n=4), which was controlled without transfusion. The delivery system was later modified and in its current incarnation has not caused any further episodes of detachment of the distal tip.
Major complications
These events had important clinical implications either in the form of haemodynamic instability or required emergent or elective surgery.
Procedural complications
Stent dislodgement and instability during the procedure occurred in 2 patients requiring surgical explantation of the stent and with insertion of homograft. Both patients had outflow tract characteristics which prevented safe anchoring of the device. One patient had a homograft too large (24 mm) for the current device and the other had a non-circumferential homograft patch used to augment the right ventricular outflow tract. There was no haemodynamic instability related to these events and the surgery was uneventful with a successful outcome.
Homograft rupture caused haemothorax in a patient which was treated acutely with auto-transfusion. Surgical exploration was required to control bleeding by placing haemostatic sutures on the homograft but the valve remained competent and was not explanted. Unusual anatomy leading to compression of left coronary artery resulted in ischaemic left ventricular dysfunction requiring cardiopulmonary resuscitation in on patient. During cardiac massage, the stent got compressed and moved away from the posteriorly placed left coronary artery with re-establishment of flow and recovery of left ventricular function. Emergency surgery was then undertaken to explant the valve and insert a homograft.
Device-related complications
The most common consequence of this group of complications was residual or restenosis. The “hammock effect” resulted from the hanging-in of the walls of the bovine venous valve within the length of the stent. The first design had the vein sutured only at its ends to the stent, leaving the wall unsupported. Before we understood the mechanism leading to his problem, 3 patients had surgical explantation of the valve and insertion of a valved conduit. Lately, we have implanted a second valve inside the first (“Russian doll concept”) and successfully dealt with the re-stenosis.
Stent fractures were seen in 8 patients (Figure 1).
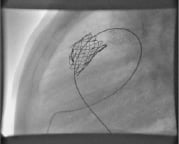
Figure 1. Stent fracture at the proximal end leading to restenosis.
Most commonly, this lead to restenosis at the level of the valved-stent, and in patients with significant gradients, a second valve was successfully implanted within the first (n=2). In one patient, the stent fractures were identified when the patient presented with clinical signs suggestive of pulmonary embolism. On chest radiography, the stent had embolised to the right pulmonary artery. The valve stent was surgically removed and a homograft was inserted a week after the event.
Endocarditis was encountered during follow-up and needed explantation of the valve in 2 patients for failure of medical therapy.
Restenosis was one of the common indications for re-intervention. Other than those from hammock effect and stent fracture, residual stenosis was seen when the valve was implanted within a small conduit (16 mm or less), or the stent could not be opened to its diameter due to external constraint from retro-sternal position (Figure 2).
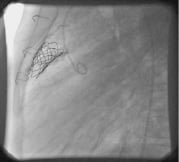
Figure 2. Residual stenosis due to a retro-sternal conduit causing external constraint.
Four patients required explantation of the valve, one interestingly had intravascular haemolysis.
Discussion
Our approach to the complications seen with this new technique was analytical and resulted in modification of the device and delivery system, better selection of patients, rigorous follow-up to detect clinically in significant events and initiating further research into refining the indications.
The delivery system in its current incarnation has not had problems with embolisation of the its distal components. The flexibility and length of the delivery system have allowed its use for delivering the device in adults and through non-femoral approach.
Patient selection is by far the most important step in trying to ensure a successful outcome and its importance cannot be overemphasized. Homograft outflow tracts are better anchoring substrates. Any homograft between 16 and 22 mm dimension at the time of implantation would be a suitable substrate. Calcified homografts invariably are smaller in dimension compared to their initial size at implantation. Severe calcification may prevent adequate dilatation of homografts and may need predilatation or stenting before pulmonary valve implantation. Non-calcified homografts may sometimes dilate and may be larger than their dimension at implantation.
Coronary compression was anticipated and details of coronary anatomy sought in every patient form early investigations and imaging and corroborated with the operation notes. We proceeded to implant a valve in one patient despite knowledge of unusual coronary anatomy as a bare metal stent had already been placed at a prior procedure within the conduit without haemodynamic compromise. Unfortunately, after implantation of the valve, left coronary compression could not be avoided.
We have now included selective coronary injection during balloon inflation in the RVOT in our protocol when the anatomy indicates possibility of coronary compression.
The diameter of Right Ventricular Outflow Tract (RVOT) measured by echocardiography, magnetic resonance imaging or angiography provide a static measurement. Enlarged RVOTs tend to be very dynamic and have different dimensions in systole and diastole. The distensibility of the RVOT to dimensions which may not anchor the valved-stent may be one of the reasons for instability and embolisation.
Mathematical modelling to predict the behaviour of the stent within different outflow tracts is being explored. This may further help in predicting successful outcomes more reliably (Figure 3).
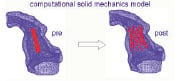
Figure 3. Mathematical modelling to predict stent behaviour inside right ventricular outflow tract.
Endocarditis is an expected complication for all implants. It is important to emphasize prophylaxis and refrain from implanting devices in acute or unresolved infection.
Stent fractures are common for all intracardiac or intravascular metallic implants3. Better understanding of metal characteristics, stress and fatigue is required to reduce the incidence and severity of this unavoidable consequence.
Despite all the complications seen with this new technique, we have had no mortality in this series, from the first case to the latest long term follow-up. We have refined various aspects of this procedure over the course of our clinical experience. With further research, we hope to expand the indications to include patients with enlarged RVOT dimensions who form a large cohort of adults with pulmonary regurgitation, currently requiring surgery. We have no doubt that this patient cohort would soon be accessible to percutaneous pulmonary valve implantation.

