Introduction
Carotid artery stenting (CAS) is a less invasive procedure for the treatment of extracranial bifurcation carotid artery stenosis, and might be an alternative to CEA in patients at high risk for peri-operative complications1.
Nevertheless, the “Achilles’ heel” of CAS is represented by the risk of cerebral embolic complications, possibly leading to major neurologic adverse events.
The availability and appropriate selection of cerebral protection devices, together with the experience of the operators, are key factors in the achievement of a low rate of complications, as showed in single-centre series2,3.
After a brief review of the rationale for cerebral protection and distal protection devices, we will deal with proximal endovascular clamping systems, recently introduced (Mo.Ma, Invatec, Roncadelle, Italy) or re-designed (NeuroProtection System, Gore, Flagstaff, AZ, USA), which seems to be highly effective in the prevention of embolic complications.
Rationale for cerebral protection
Cerebral anti-embolic protection is a crucial issue in the modern era of carotid artery stenting (CAS). There are three main families of devices: distal occlusion balloons, distal filters and proximal occlusion balloons with or without flow reversal. Although no randomised trial has formally compared unprotected versus protected CAS, both experimental and clinical data strongly suggest that cerebral protection is mandatory4,5.
Recent literature data regarding cerebral protection during CAS report some interesting points:
1) visible debris, mainly represented by atheromatous plaque material, was observed in 60% of cases of filter-protected CAS by Sprouse et al6 and in 66.8% by our group 3, with particles > 2 mm in 9% of cases;
2) in the German registry, the use of an embolic protection device was associated with a significantly lower rate of ipsilateral stroke (1.7% vs 4.1%; p=0.007)7;
3) we reported a 79% reduction in the rate of embolic complications with the use of cerebral protection8;
4) in the early phase of the EVA-3S study unprotected CAS was associated with a 3.9 times higher stroke rate at 30 days as compared to CAS performed under cerebral protection9;
5) a systematic review by Kastrup et al conducted on more than 3,000 patients showed that cerebral protection significantly reduced the rate of neurological complications as compared with unprotected CAS (1.8% vs 5.5%, respectively; p<0.001)10;
6) a 2003 review of the global carotid artery stent registry found that the rates of stroke and death were 5.2% for unprotected CAS and 2.2% for protected CAS11.
According to the size, embolic particles can be distinguished in macroemboli (> 100 µm) and microemboli (< 100 µm).
Macroemboli, especially > 200 µm in size, are usually associated with clinically evident neurological damage, ranging from transient ischaemic attack to major stroke; the effects of microembolisation, on the contrary, are not well known and may include subtle changes in neurocognitive function rather than motor or sensory manifestations12.
Indeed, athero-embolisation can initiate an inflammatory process that eventually leads to cellular infiltration and fibrosis, and the link between occult cerebral injury causing cognitive deficit and embolisation of particles 10-70 µm in size has been established following cardiopulmonary bypass13.
Surrogate markers of cerebral embolisation, such as microembolic signals (MES) detected by trans-cranial doppler (TCD), have shown that this event, although more frequent during stent delivery and postdilatation, may occur in each procedural step of CAS14; therefore the ideal cerebral protection device should be able to avoid both macro- and microembolisation from the beginning to the end of the procedure.
Distal protection devices
Distal protection devices are placed in the ICA distally to the lesion and include occlusive balloons and filters.
Distal balloons, actually the first protection device to be introduced15,16, have the limitation of unprotected crossing of the lesion and, despite being able to block microemboli by occluding the ICA, can lead to cerebral embolisation through collaterals from the external carotid to the middle cerebral artery (retinal and cerebral infarcts through large peri-orbital and occipital collaterals have been reported)17,18. Moreover, about 5 to 8% of patients develop clamping intolerance19.
Distal filters are nowadays used in about 90% of CAS procedures, mainly because they are quite easy to use and intuitive for beginners, allowing the maintenance of cerebral perfusion.
Nevertheless, these devices have some drawbacks:
– they are not effective in trapping microemboli, since the pore size ranges from 100 to 140 µm;
– the lesion must be crossed by the wire and the filter itself, with the risk of unopposed embolisation, especially in tight lesions;
– even macroemboli may pass in the case of incomplete vessel wall apposition (tortuous landing zone or large artery);
– emboli may be dislodged during the retrieval of filters (squeezing effect).
Finally, both distal balloons and filters may be an embolic source themselves, due to intimal damage at the level of the ICA landing zone20. In some studies, the use of distal filters was even associated with a greater MES count as compared to unprotected stenting, although this did not translate in a clinically evident difference21.
Proximal protection devices
These devices work by interrupting or reversing blood flow in the ICA.
As compared with filters, they offer the advantage of crossing the lesion under protection with the preferred guidewire, blocking both macro- and micro- emboli irrespective to size.
Moreover, navigation of the device in the distal ICA is not required, thus reducing the risk of intimal damage, spasm or dissection.
Nowadays there are two proximal protection devices available: the NeuroProtection System (NPS, Gore), which derives from the Parodi anti-embolic system (PAES, ArteriA), and the Mo.Ma system (Invatec).
NeuroProtection System (NPS, Gore, Flagstaff, AZ, USA)
This system (figure 1 and 2), allows complete arrest of ICA flow, continuous passive ICA flow reversal or augmented active ICA flow reversal.
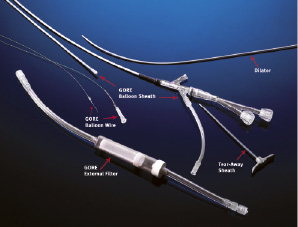
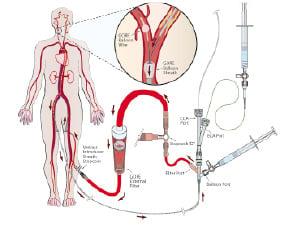
Figure 1. Upper panel: schematic representation of the Neuro Protection System device (Gore, Flagstaff, AZ, USA). Lower panel: flow reversal is achieved at the treatment site by selectively occluding common carotid and external carotid artery. An arterio-venous shunt is established with the femoral vein and embolic particles are captured in the external filter. (Reproduced with permission from the company).
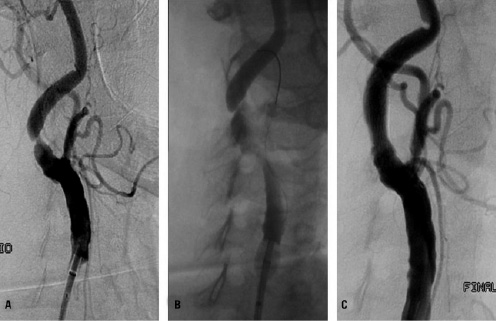
Figure 2. Protected carotid artery stenting in an 87-years old patient. Panel A: digital subtraction angiography showing critical stenosis of the right internal carotid artery. Panel B: endovascular clamping with the NPS device is obtained by inflating the balloons in the external and in the common carotid artery. The injection of dye shows flow arrest/reversal. Panel C: positioning of a self-expandable, tapered, nitinol stent with combined cell design (open cells at the edges and closed cells in the middle): 10.0-7.0/40 mm Cristallo Ideale stent (Invatec, Roncadelle, Italy).
This is operated by endovascular clamping of the common carotid artery – by inflating a balloon located at the tip of an 11 Fr sheath (9 Fr in the new NPS system) – and of the external carotid artery – by inflating an independent balloon catheter advanced in the external through the sheath. At this point, blood flows in the sheath outside the body, driven by cerebral back pressure (passive flow) or actively aspirated by a syringe, and re-enters the body through a 6 Fr venous sheath placed in the femoral vein. A filter (pore size 180 µm) collects debris before the blood re-enters the venous system, in order to avoid the risk of paradoxical embolism in case of a patent foramen ovale.
Once that the system is working, the lesion can be crossed with a guidewire under protection and angiographic guidance, since the contrast medium is cleared by backflow; then, conventional stenting and postdilatation are performed. After each stage of the procedure, particularly those associated with the greatest risk of embolisation, 10 ml of blood are actively aspirated, then the balloons are deflated while active suction is applied to retrieve any particle contiguous to the balloon occluder.
Most of clinical data about this device were obtained with the first version, i.e. the PAES.
In the first series of 100 patients reported by Parodi in 2001, no embolic stroke was observed, but clamping intolerance occurred in 8%22. Other small, non-randomised studies confirmed the efficacy of PAES in preventing embolic complications23,24. In 2005, Parodi reported about the first 200 patients treated; in this series, the technical success rate was 98.5%, the thirty-day stroke and death rate was 1.5% and perioperative clamping intolerance was observed in 6 patients (3%)25.
Mo.Ma (Invatec, Roncadelle, Italy)
The Mo.Ma system (figure 3 and 4), is made by a 100 cm, 9 Fr catheter with a 6 Fr working channel and two distal, independently inflatable balloons placed at the distance of 7.2 cm.
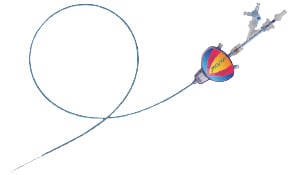
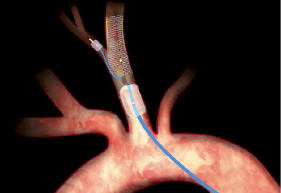
Figure 3. Left panel: schematic representation of the Mo.Ma device (Invatec, Roncadelle, Italy). Right panel: flow arrest is achieved by inflation of the two balloons; the procedure is performed through the 6 F working channel; at the end of the procedure the static blood column is aspirated with a syringe, filtered and discarded. (Reproduced with permission from the company).
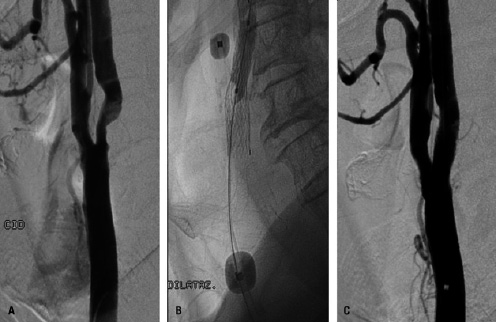
Figure 4. Protected carotid artery stenting in a 67-years old patient. Panel A: digital subtraction angiography showing critical stenosis of the right internal carotid artery. Panel B: endovascular clamping with the Mo.Ma device is obtained by inflating the balloons in the external and in the common carotid artery. The injection of dye shows flow arrest. Panel C: positioning of a self-expandable, tapered, nitinol stent with combined cell design (open cells at the edges and closed cells in the middle): 10.0-7.0 / 40 mm Cristallo Ideale stent (Invatec, Roncadelle, Italy).
The distal balloon occludes the external carotid artery up to a diameter of 6 mm, whereas the proximal balloon occludes the common carotid artery up to a diameter of 13 mm, determining a static blood column at the carotid bifurcation.
At this point the stenting procedure can be performed by crossing the lesion under protection with the selected materials; after postdilatation, at least three 20 ml syringes of blood are actively aspirated and checked for debris with a filter before deflating the balloons.
The first clinical experience with the Mo.Ma system was reported by Diederich et al26, who treated 42 patients (26.2% with symptomatic carotid artery disease) with an overall technical success of 97.6%. Mean clamping time was 10.6±6.5 min, transient clamping intolerance occurred in 12% of patients, macroscopic debris were collected in 76,1% of cases; two patients had neurological deficits that lasted 2 and 12 h, respectively, and two other patients (4.7%) had a minor stroke.
In the PRIAMUS multicentre registry27, 416 patients (63.4% with symptomatic carotid artery disease) underwent CAS with the Mo.Ma device. Technical success was achieved in 99% of cases, mean clamping time was 4.91±1.1 min, transient clamping intolerance was observed in 5.76%, whereas macroscopic debris was retrieved in about 60% of patients.
At thirty day follow-up, the cumulative incidence of adverse events was 4.56%, with a 0.72% rate of major strokes and deaths.
The efficacy of the Mo.Ma device in preventing microembolisation was assessed in a comparative study with a distal filter (E.P.I. FilterWire, Boston Scientific) by detecting microembolic signals with transcranial Doppler during the CAS procedure28.
The authors identified five different procedural steps: 1) positioning of the protection system; 2) lesion crossing; 3) stent deployment; 4) balloon dilatation; 5) retrieval of the protection system. The number of MES was significantly lower in steps 2 to 4 with the Mo.Ma as compared with the filter, whereas no significant differences were observed during the first and last phase.
MES detection is a surrogate marker of cerebral microembolisation which can be hampered by technical limitations, such as the inability to differentiate between solid and gaseous emboli29; nevertheless, the association of an high MES count with neurological complications was established in the Antonius CAS registry9.
Limitations of proximal protection devices
The drawbacks of proximal protection devices include their large size, the occurrence of clamping intolerance and the impossibility of their use in patients with severe disease of the external or of the common carotid artery, whereas contra-lateral ICA occlusion is not necessarily a contraindication.
The need for large femoral sheaths may preclude the insertion of the devices in patients with severe peripheral diffuse disease and could theoretically be associated with an increase in vascular access site complications. Yet, with the first version of the Mo.Ma device, requiring a 10 Fr femoral sheath, in the PRIAMUS registry20 the rate of local complications was 4.08%, none of which required surgical repair or blood transfusions. Higher complication rates were reported by Rabe et al30 with the PAES, but, given the current availability of 9 Fr size for both the Mo.Ma and the NPS device, it is reasonable to expect a low rate of clinically significant access site complications in the future.
Clamping intolerance may occur in a definite portion of patients (up to 8%) following interruption of brain perfusion during the intervention and is generally associated with severe contra-lateral disease or poorly developed cerebral collateral circulation. An intraprocedural parameter predictive of tolerance is represented by a back pressure > 30 mmHg. Another key factor is overall clamping time, that has progressively shortened with the increased experience of operators (from 10 minutes in the study of Diederich19 to 5 minutes in the PRIAMUS registry20, with a parallel decline in the rate of clamping intolerance from 12% to about 6%15,18).
The same holds true for the PAES/NPS device, since the rate of clamping intolerance dropped from 8% in 2001 to 3% in 2005.
However, the occurrence of clamping intolerance does not represent an absolute contraindication to carry on the procedure. Indeed, three strategies can be adopted: hurry up in order to restore perfusion as soon as possible; positioning under protection a distal filter and then deflating the balloons (“seat-belt and air-bag” technique)31; perform a step by step procedure in which the balloons are inflated and deflated at each procedural step.
Finally, for both proximal protection devices two potential drawbacks have to be considered:
1) a non-occlusive balloon or a superior thyroid artery originating proximal to the ECA balloon may lead to continuous antegrade flow in the target vessel during clamping;
2) at the end of procedure, the balloon in the ECA, usually jailed by the stent, must be removed and, at least theoretically, might be entrapped in the struts of an open cell geometry stent.
Conclusions
At this time large, clinical studies comparing proximal with distal protection are lacking, so device selection is quite empirical.
In challenging anatomies, with angulated ICA-CCA take-off and/or lack of a suitable ICA landing zone for distal protection, the use of proximal protection should be strongly recommended.
The same holds true for lesions with high embolic risk, since proximal protection devices seem to be more effective than filter in avoiding distal embolisation, especially in the procedural steps at higher risk and irrespective of debris size.
Therefore, non-invasive characterisation of the carotid plaque in order to quantify its embolic potential (“vulnerable plaque”) should represent a very important issue when planning the CAS procedure.
Both long lesions and clinically unstable plaques (i.e. recurrent TIAs) define a high risk lesion subset, because of high plaque burden and inflammatory activation, respectively.
Indeed, Krapf et al32 reported that the risk of new cerebral ischaemic lesions at diffusion-weighted MRI after CAS was related to the length of lesion as assessed by B-mode echography, whereas preprocedural leukocyte count was found to be associated with increased microembolisation during CAS33.
The vulnerable plaque, as opposed to the stable, fibrous plaque, is made of a large lipid pool covered by a thin fibrous cap.
A study in 200 CEA specimens showed that plaque phenotype correlated with embolisation during carotid endarterectomy, since vulnerable plaques were more prone to cause perioperative microembolisation as compared to fibrous plaques34.
Fatty, vulnerable plaques are less echogenic, and this pattern can be quantified by the Grey Scale Median (GSM) method. In the ICAROS study35, the risk of CAS-related stroke was 7.1% in lesions with GSM < 25 and 1.5% in lesions with GSM > 25.
Therefore clinical, biochemical and morphological data, many of which are probably still unknown, should be assessed and integrated in order to predict the embolic risk of a specific carotid lesion during CAS.
If this risk is estimated to be high and there are no anatomical contraindications, proximal protection would probably represent the safest approach for the patient.

