Abstract
Background: The ACHIEVE™ Paclitaxel Eluting Coronary Stent System (CSS) is a non-polymeric paclitaxel coated stent loaded with a dose density of 3.0 µg/mm2 stent surface area. DELIVER II set out to evaluate the use of this stent in the treatment of patients with coronary lesions with a higher risk of revascularization including chronic total or sub-total occlusion, small vessel, bifurcated, and long lesions, multivessel disease, and restenotic lesions.
Methods: DELIVER II was a prospective, non-randomized, single-arm, multi-centre study. A total of 1531 patients with 1986 lesions were enrolled at 86 sites worldwide. The primary endpoint was target lesion revascularization (TLR) rate at six months follow-up. The lesion subsets were comprised of 28.5% restenotic lesions, 16.5% chronic total or subtotal occlusions, 29.0% bifurcation lesions, 37.4% small vessels and 16.6% long lesions.
Results: The Target Lesion Revascularization (TLR) rate (intent-to-treat) at 180 days was 8.2% (156/1909). The MACE rate for the overall per-protocol population was 3.6% (47/1310) at 30 days, 13.0% (167/1287) at 180 days, and in a sub-set of 500 patients, 20.5% (84/409) at 365 days. Multivariable logistic regression analysis identified that the factors leading to higher risk of revascularization were left anterior descending artery lesions, restenotic lesions, the post-procedural minimum luminal diameter, total stent length and number of diseased vessels.
Conclusion: DELIVER II demonstrated comparatively low rates of TLR and MACE for the ACHIEVE™ Paclitaxel Eluting CSS in high risk patients. In addition, this registry helped to identify risk factors leading to an increased risk of revascularization in difficult-to-treat patient groups.
Recent studies evaluating the local application of anti-proliferate drugs have shown that this treatment strategy successfully inhibits or reduces restenosis1-6. However, these studies were performed in patients with simple coronary lesions. Patients, including diabetics7, with more complex lesions such as in-stent restenosis, long diffuse coronary stenosis8-10, lesions involving bifurcation11, chronic total occlusion12, and multivessel disease13 have a higher risk of restenosis and the effects in these patients of different drugs and methods of delivery still needs to be evaluated. Therefore, we evaluated the delivery of paclitaxel on a non-polymer coated stent in the treatment of complex coronary artery lesions.
Methods
Study population
The DELIVER II study was a prospective, non-randomized, multi-centre evaluation, which recruited 1531 patients with 1986 target lesions at 86 sites across Europe, Middle East and South Africa. The medical ethic committees at the investigational sites approved the study protocol, and written informed consent was obtained from all patients.
Major inclusion criteria were patients with complex lesions such as chronic total or sub-total, restenotic or bifurcated lesions. Patients had to have one of the following: 1. one single complex target lesion (being either a chronic total occlusion, a sub-total occlusion (TIMI 1), a restenotic lesion (including in-stent restenosis) or involving a bifurcation site) with a length ≤ 25 mm (not a de novo lesion), 2. two complex target lesions with a length ≤ 25 mm (de novo lesions allowed), 3. one complex lesion with a length > 25 mm (de novo lesions allowed).
Patients exclusion criteria were: having more than one lesion to be treated in the same vessel, having more than two lesions to be treated, and previous intracoronary brachytherapy or drug eluting stent treatment.
Study stent
The stent used in the DELIVER II study was the ACHIEVE™ Paclitaxel Eluting CSS stent (Guidant Santa Clara, CA), which incorporated a non-polymeric coating of paclitaxel (3.0 µg/mm2). With this stent design, the drug was gradually released between 4 hours and 14 days after implantation (non-linear release fashion), with an overall release rate of approximately 3.5 µg/day. The stent was available in lengths of 8, 13, 15, 18, 23, and 28 mm and diameters of 2.5, 3.0, 3.5, and 4.0 mm.
Study endpoints
The primary endpoint for the study was the Target Lesion Revascularization (TLR) rate at 180 days following index procedure, in order to reflect clinical practice and clinical impact on patients. The secondary endpoints included Target Vessel Failure (TVF) rate at 180 days following index procedure, Major Adverse Cardiac Events (MACE) rate at 30 days and 180 days following index procedure, the MACE rate at 1-year in a subset of 500 patients (first 500 enrolled patients), device and procedure success. All endpoint related events were adjudicated by an independent clinical event committee. Lastly, a multivariable logistic regression was performed to identify risk factors, which might contribute to TLR at 180±20 days.
Definitions
MACE was defined as the occurrence of death (all causes), Q-wave or non-Q-wave Myocardial Infarction (MI), or TLR by Coronary Artery Bypass Surgery (CABG) or Percutaneous Coronary Intervention (PCI), with non-Q MI being defined as CK enzyme elevations of more than 3 times the upper limit of normal and presence of CK-MB. TVF was a composite of MACE and target vessel revascularization by CABG or PCI. Subacute stent thrombosis was any unexplained cardiac death < 30 days post index procedure, any subacute closure (outside of cath lab) requiring revascularization of the target site < 30 days, any total closure indicated by quantitative coronary angiography < 30 days. Device success (per lesion) was achieved when there was a final result of < 50% stenosis post-procedure of the target lesion using the first-intended treatment device. Procedural success (per patient) was defined as a final result of <50% residual post-procedure of the target lesion using the first-intended treatment device and/or any adjunctive device including additional stents without death, emergent bypass surgery or Q-wave or non Q-wave MI post-procedure prior to hospital discharge. Small vessels were defined as ≤ 2.75 mm as reference diameter, long lesions were defined as target lesion length >25 mm.
Procedural characteristics
All patients received a loading dose of aspirin and clopidogrel up to 300 mg within 24 hours prior to the procedure. During the procedure, appropriate anticoagulation therapy with intravenous unfractionated heparin was given to the patients. Following intracoronary injection of nitroglycerin (100-200 µg), baseline angiography of the target vessel was completed as per Angiographic Core Laboratory protocol. The target lesion had to be pre-dilated with an appropriately sized balloon. If post-dilatation was required, the balloon position had to be confined carefully within the stent boundaries. Post procedure, the patients were maintained on 75 mg clopidogrel daily for 3 months (common practice at that time for drug eluting stents) and aspirin (dosage according to hospital practice) indefinitely.
Follow-up
A clinical follow-up was performed at 30 days and 180 days post-index procedure, and at 365 days in a subgroup. There was no scheduled angiographic follow-up per protocol. The Angiographic Core Laboratory for evaluation of the baseline and post-interventional angiograms was Heart Core BV (Leiden, The Netherlands). Cardiac enzymes, CK and CK-MB (if CK was elevated) and troponin were routinely measured between 12-hours and 24-hours post-index procedure. ECG was performed immediately post procedure as well as after 12 to 24 hours.
Statistical analysis
The study sample size was driven by the ability to detect a composite 180 days TLR rate that was less than or equal to the expected Objective Performance Criteria (OPC) of 10% TLR (CABG and PCI) rate at 180 days follow-up. The OPC was calculated using Clopper Pearson’s upper 95% C.I of the point estimate of the 180-day TLR rate (6.1%) observed in the PENTA Registry. Therefore, with a power of 95% and alpha of 5%, 1374 patients were required to demonstrate that the 180-day TLR rate is less than or equal to the OPC. The primary endpoint was reviewed on an intent-to-treat basis. The secondary endpoints were reviewed on a per-protocol basis (this is the total of patients who have received the study device at the target lesion(s), and who have no major protocol deviations) taking into account the time windows allowed for the different follow-up visits. The subject population for each of the high-risk subgroups (de novo lesion, restenotic lesion, chronic total/subtotal occlusion, bifurcation lesion, small vessels ≤ 2.75 mm and long lesions >25 mm) included a minimum of 100 subjects. To study the relationship between TLR and various prognostic factors, logistic regression techniques were used.
Results
A total of 1531 patients with 1986 lesions were included in the study between April 2002 and September 2002. 221 patients did not receive treatment according to the protocol so these patients were excluded from the per-protocol analysis. The two main reasons for being excluded from the per-protocol analysis are 1. single de novo lesions without complex characteristics and 2. two lesions treated in the same vessel. Therefore the per-protocol population consisted of 1310 patients having 1676 lesions.
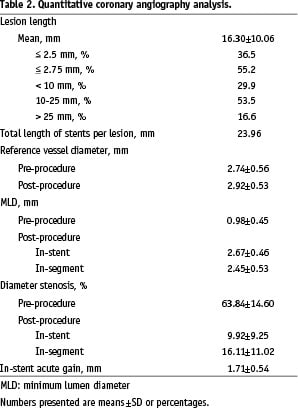
A summary of the baseline patient and lesion characteristics is shown in Table 1, the results of the baseline and post-procedural quantitative coronary angiography (QCA) analysis are shown in Table 2.
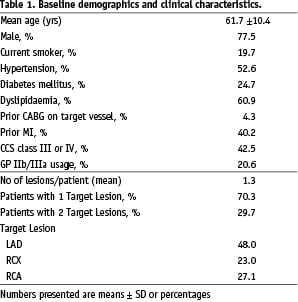
The lesion subsets were comprised of 28.5% restenotic lesions, 16.5% chronic total or subtotal occlusions, 29.0% bifurcation lesions, 37.4% small vessels and 11.2% long lesions. The mean lesion length was 16.3 mm; 53.5% of the lesions had a length of 10-25 mm.
The overall device success rate for the per-protocol population was 99.5%. The procedure success rate was 97.3%. The total length of stents implanted per lesion was 23.96 mm, as measured by QCA.
The mean percentage diameter stenosis was 63.84±14.60% pre-procedure and 16.11±11.02% post-procedure (in-segment). The overall in-stent acute gain was 1.71±0.54 mm (Table 2).
Clinical results
The non-hierarchical TLR rate at 180±20 days on the intent-to-treat population, which was the primary endpoint of the study, was 8.2% (TLR-PCI 6.8%, TLR-CABG 2.0%).
The secondary endpoint of MACE was 13.0% at 6 months for all patients, and in the subgroup only 13.7% at 6 months and 20.5% at 1-year (Table 3).
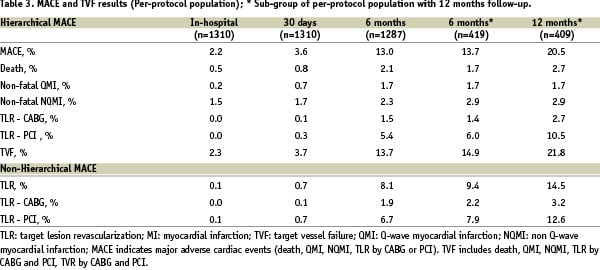
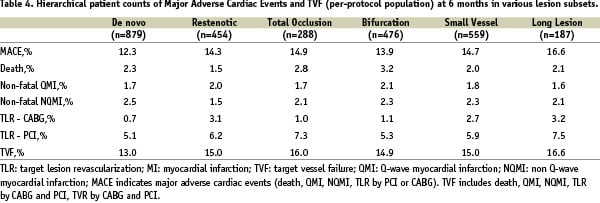
The MACE results of the study subgroups are listed in Table 4.
In the sub-group of 500 patients who had follow-up at 1-year, the overall non-hierarchial TLR rate, per-protocol, at 6 months was 9.4%; (TLR PCI 7.9%, TLR CABG 2.2%). In this same group at 1 year, the TLR rate was 14.5% (TLR-PCI 12.6%, TLR-CABG 3.2%) (Table 3). The stent thrombosis rate at 30 days was 0.9%.
Multivariable logistic regression
A multivariable logistic regression was performed on the intent-to-treat population; included in this analysis are demographic, clinical, angiographic, and procedural variables. The analysis showed 5 significant independent risk factors, which have a higher risk for TLR at 180 days. The risk factors identified were LAD lesion, restenotic lesion, post-procedure MLD, number of diseased vessels and total stent length (Figure 1).
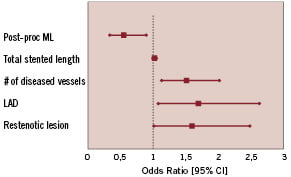
Figure 1. Results of multivariable analysis showing risk factors contributing to a higher risk of revascularization at 180±20 days after implantation of an ACHIEVE paclitaxel-eluting stent.
Discussion
Compared with previous trials, DELIVER II recruited the largest population of patients with high risk of restenosis, e.g. 24.7% of the patient population was diabetic and of the lesions treated, 28.5% were restenotic, 16.5% chronic total or subtotal occlusions, 29% bifurcation lesions and 37.4% were small vessels. The intention of this study was to demonstrate the effect of this stent design in an ‘all high-risk-comers’ population.
Despite this high-risk patient cohort, the use of the non-polymer-based ACHIEVE™ Paclitaxel Eluting CSS in DELIVER II resulted in a TLR rate of 8.2% at 6-month follow-up. In addition, a MACE rate of 13.0% at 6 months in DELIVER II was a strong indicator for device safety. However, it needs to be considered, that there was only a clinically driven angiographic follow-up which may have affected the total TLR rate.
The multivariable logistic regressions identified lesion and patient risk factors, which might lead to a higher TLR rate, including LAD lesion location, restenotic lesions, post-procedure MLD, number of diseased vessels and stent length. Therefore, patients receiving non-polymeric paclitaxel eluting stents that have one or more of the risk factors as described above, should be followed up more extensively. Remarkably, diabetes was not a predicted risk factor for TLR at 180 days.
In summary, DELIVER II demonstrated the mid-term safety of the non-polymer based ACHIEVE™ Paclitaxel Eluting CSS design as well as its interaction with specific lesion and patient characteristics on the clinical outcome.
However, the efficacy of this stent observed in DELIVER II needs to be set alongside the apparent lack of significant efficacy observed in DELIVER I. The randomized DELIVER I trial evaluated the same ACHIEVE stent for treatment of focal de novo lesions compared to a bare metal control stent14. At 9 month follow-up, there was only a trend to a reduction of the primary endpoint of target vessel failure without statistical significance (11.9% for ACHIEVE stent versus 14.5% for bare metal control stent, p=0.13). The in-stent binary restenosis rate at 8 months was 14.9% for the ACHIEVE stent versus 20.6% for the control (one-sided p=0.076) with a significantly reduced in-stent late loss of 0.81 mm for the ACHIEVE stent versus 0.98 mm for control (p=0.0031). However, compared to late loss values of other drug eluting stents (0.11 mm for ELUTES, 0.39 mm TAXUS IV, 0.17 mm SIRIUS)6,15,16 the late loss results of the ACHIEVE drug eluting stent were fairly high.
Since the efficacy of paclitaxel itself has been shown in several studies, potential reasons for the observed late loss might be the lack of a polymer controlled drug release. In addition, as compared to the non-polymer based ASPECT and ELUTES studies15,17, which achieved lower late loss values than DELIVER I without having a polymer, the design of the bare metal stent platform of the ACHIEVE™ Paclitaxel Eluting CSS was different, probably resulting in different abluminal stent surface areas for each stent. This might cause differences in drug distribution that might be less uniform than that produced by the other two stents. This would indicate that even in the drug eluting stent era the stent design itself is as important as other factors such as the drug release kinetics and the polymer used.
However, irrespective of the DELIVER I data, the DELIVER II study of >1500 patients demonstrated safety in a high risk patient population group, through low rates of clinical events at follow-up.
Conclusions
The DELIVER II study demonstrated safety of the non-polymer based Paclitaxel Eluting ACHIEVE™ CSS with a low rate of target lesion revascularizations in a complex lesion population. In addition, multivariable analysis identified several factors that increase the risk of revascularizations.
Limitations
There are several limitations of this study: 1. a high number of patients (221) were excluded due to non-compliance with the protocol. 2. Lack of angiographic follow-up may have led to a lower revascularization rate. However this study reports on the clinical event outcome in a complex lesion cohort and it could be argued that any additional lesions that may have been found during a routine angiographic follow-up are therefore clinically irrelevant. 3. The 6-month clinical follow-up was much shorter than that performed in current DES trials. This is mainly due to the fact that DELIVER II was a very early DES trial, when the need for a longer follow-up was not yet realized; and the 6 month follow-up period was still accepted as a standard interval. However, ongoing in-stent intimal proliferation might exceed the 6 month interval, particularly discussed in DES. The increase of the TLR rate between 6 and 12 months in the 12-month subgroup population might be related to this issue. 4. Lastly, DELIVER II was a registry without a control group. This limits the ability to comment on the efficacy of this device.
Appendix
Sponsor: Guidant Corporation, Santa Clara, California, USA
Principal Investigator: Prof. E. Grube (Germany)
Executive Committee: Prof. A. Bartorelli (Italy); Dr. D. Blanchard, MD (France); Dr.A. Gerschlick, MD (UK); Dr. C. Macaya, MD (Spain); C. Homsy, MD (Clinical Research Guidant Europe); Anton Van Weert, PhD (HeartCore, The Netherlands); Sophie Henry (Clinical Research Guidant Europe), Els Boone (Clinical Research Guidant Europe)
Data Safety Monitoring Board (DSMB): J. Tijssen, MD (The Netherlands); Prof. V. Legrand (Belgium); W. Wijns, MD (Belgium)
Clinical Events Committee (CEC): Prof. C. Hanet (Belgium); P. Stella, MD (The Netherlands); B. Meursing, MD (The Netherlands)
Angiographic Core Laboratory: Heart Core, Leiden, The Netherlands
Data Management: Data Coordination Centre and Site Monitoring: Guidant Europe, Diegem, Belgium
The following investigators and institutions participated in the DELIVER II trial (number of patients):
Dr. Büttner, Herzzentrum Bad Krozingen, Germany (53); Prof. Marco, Clinique Pasteur, France (51); Prof. Grube, Herzzentrum Siegburg GmbH, Germany (50); Dr. Drzewiecki, Silisean University Medical School, Poland (50); Dr. Glatt, Centre Cardiologique du Nord, France (50); Prof. Hamon, Hôpital de la Côte de Nacre CHU, France (50); Prof. Piek, Academisch Medisch Centrum, The Netherlands (50); Prof. Zeiher/Auch Schwelk, Klinikum der Johann Wolfgang Goethe Universität, Germany (49); Dr. Oemrawsingh, Leids Universitair Medisch Centrum,The Netherlands (42); Prof. Dietz, Franz Volhard Klinik, Germany (38); Dr. Morice, ICPS, France (36); Dr. Di Mario, San Raffael, Italy (35); Dr. van Boven, Groningen AZG, The Netherlands (34); Prof. Hoffmann, Med. Einrichtung der RWTH-Aachen, Germany (33); Dr. Dawkins, Southampton General Hospital, UK (31); Dr. Macaya, Clinico San Carlos, Spain (30); Prof. Schöls, Universitätsklinikum Heidelberg, Germany (28); Dr. Hennen, Universitätsklinik Homburg/Saar, Germany (28); Dr. Bartorelli, Centro Cardiologico Monzino, Italy (27); Prof. Fleck, Deutsches Herzzentrum Berlin, Germany (27); Dr. De Benedictis, Ospedale Mauriziano, Italy (24); Prof. Haase, Kardiologisches Centrum, Germany (24); Prof. Charbonnier, Hôpital Trousseau, France (23); Dr. Guenot, Clinique Belledone, France (22); Dr. Antoniucci, Azienda Ospedaliera Careggi, Italy (21); Dr. Neuzner, Klinikum Kassel, Germany (21); Dr. Bramucci, Policlinico San Matteo, Italy (20); Prof. Bonzel, Klinikum Fulda, Germany (20); Prof. Polonski, Silisean Heart Disease Center, Poland (20); Dr. Valdés, Hospital Universitario Virgen Arrixaca, Spain (20); Dr. Levy, Clinique La Valette, France (19); Dr. Wiemer, HZ Bad Oeynhausen, Germany (18); Dr. Rubino, Casa di Cura Montevergine, Italy (18); Prof. Kuck, Allgemeines Krankenhaus St. Georg, Germany (17); Prof. Lablanche, CHU Cardiologique, France (17); Dr. Berland, Clinique Saint Hilaire, France (17); Prof. Thale, Schüchtermann Klinik Bad Rothenfelde, Germany (17); Dr. Colombo, EMO-Centro Cuore Columbus, Italy (16); Prof. Jung, Städtisches Klinikum Villingen-Schwenningen, Germany (16); Dr. Bethencourt, Hospital Universitario Son Dureta, Spain (16); Prof. Spaulding, Hôpital Cochin, France (16); Dr. Banning, John Radcliffe Hospital, United Kingdom (15); Dr. Holmberg, Royal Sussex County Hospital, United Kingdom (15); Dr. Mabin, Vergelegen Medi Clinic, South Africa (15); Dr. Guerin, Clinique Parly 2, France (15); Prof. Eber, Allgemeines Krankenhaus Wels, Austria (15); Dr. Cassel, Milpark Medical Centre, South Africa (14); Dr. Fourier, Clinique La Louvière, France (14); Dr. Zelizko, Ikem, Czech Republic (14); Prof. Erne, Kantonsspital Luzern, Switzerland (13); Dr. Levy, Wythenshawe Hospital, South Manchester University Hospitals NHS Trust, United Kingdom (13); Prof. Peiffer, Universitätsklinik Leipzig, Germany (13); Dr. Blanchard, Clinique Saint Gatien, France (12); Dr. Thomas, King’s College Hospital, United Kingdom (12); Dr. Fontanelli, Presidio Ospedaliero di Vicenza, Italy (12); Prof. Cassagnes, CHU Clermont-Fernand, France (11); Dr. Makowski, Clinique Ambroise Paré, France (11); Dr. Esplugas Oliveras, Hospital Principes De Espana, Spain (11); Prof. Klein, University Hospital Graz, Austria (10); Prof. De Servi, Ospedale Civile di Legnano, Italy (9); Dr. Gerschlik, Glenfield Hospital, United Kingdom (9); Prof. Lotan, Hadassah University Hospital, Israel (9); Dr. Betriu, Hospital Clinico y Provincial de Barcelona, Spain (8); Dr. Thuesen, Skejby Sygehus, Denmark (8); Dr. Khalife, CH Metz-Thionville, France (8); Prof. Eberli, Universitätsspital Zürich, Switzerland (7); Prof. Carrié, CHU Rangueil; France (7); Dr. Reimers, Ospedale Civile, Italy (6); Prof. Rothman, The London Chest Hospital, United Kingdom (5); Dr. Sionis, 1st IKA Hospital “Penteli”, Greece (5); Dr. Ettori, Spedali Civili di Brescia, Italy (4); Prof. Mäurer, Klinikum Bayreuth, Germany (4); Dr. Tsikaderis, Saint Luke’s, Greece (4); Prof. Danchin, Hôpital Européen Georges Pompidou, France (3); Dr. Medina, Hospital Dr. Negrin, Spain (3); Dr. Verheye, Middelheim Ziekenhuis, Belgium (2); Dr. Janssens, Imelda Ziekenhuis, Belgium (1).

