Abstract
The treatment of coronary artery disease underwent a remarkable shift over the past decades. Such progress would not be possible without the ingenuity of entrepreneurial scientists, sound clinicians and the courage of our patients. This article reports the two events that marked the beginning of modern interventional cardiology: the implantation of the first balloon-expandable stent (Palmaz-Schatz™), and the first sirolimus-eluting stent (Cypher™) in human coronary arteries. Both procedures were performed, almost a decade apart, at the Institute Dante Pazzanese of Cardiology in Sao Paulo, Brazil.
We were asked to provide a historical description of the first experiences with balloon expandable stents, i.e. bare metal and drug-eluting stents. The courage and ingenuity of pioneers such as Andreas Gruentizg, Julio Palmaz and Richard Schatz paved the way for what would soon become the preferred coronary revascularization strategy, namely intracoronary stents. In 2003, an estimated 1,244,000 PCI versus 467,000 bypass procedures were performed in the United States1.
Our group had the privilege to implant the first balloon expandable stent (Palmaz-Schatz™; Cordis, Warren, NJ, USA) in a human coronary artery in 1987. More recently, our group was once again honored to conduct the first-in-man (FIM) experience with sirolimus-eluting stents (Cypher™, Cordis, Warren, NJ, USA). These memorable experiences will be reported in this article.
The Palmaz-Schatz™ Stent FIM experience
We were initially approached by Johnson and Johnson regarding the possibility to test a stainless steel balloon-expandable tubular fenestrated prosthesis in a human coronary artery. Dr Richard Schatz provided some preliminary data and technical explanations of what would soon be named the Palmaz-Schatz stent2. Despite the lack of strong regulatory agencies at the time, the ethical principles, which are an inherent part of medicine, forced us to repeat and practice the implantation procedure in the experimental laboratory at the Institute Dante Pazzanezze of Cardiology before any attempt was made to treat humans. Meanwhile, we were supposed to select the candidate for this first experiment, who needed to have a well collateralized single vessel coronary disease. As you can imagine, it was no trivial task to explain to a patient that he would undergo a procedure that had not been done in humans and that we really didn’t understand the risks associated with it, while coronary artery bypass surgery was already a well establish therapeutic options for such patients. We certainly could not have predicted that stents could be associated with a 5 to 20% risk of thrombosis. Had we known better, such an experience would likely have been delayed for years or even decades, as it took almost 10 years to find the solution to the problem of stent thrombosis3. We did not know much about the potential benefits either. Nevertheless, we were able to identify the candidate and schedule what would represent the starting point of a new era in interventional cardiology. Luckily, the patient was a dental prosthesis professional, which made it easier to explain the concept of intracoronary stent –it is likely that he knew more about “stents” than us. He was 56 years old at the time, and had a history of dyslipidaemia and smoking. He had a severe stenosis in the right coronary artery and a well developed collateral from the left system. However, it took a while before we could perform the procedure, as the cine-angiograms were sent to the United States for evaluation and approval. Then, in December, 1987 after we had done 3 animal implants together with Dr Schatz, the patient returned for his procedure. Unfortunately the right coronary artery was found to be completely occluded and we had to make a decision on whether to proceed with the experiment or find another candidate. Once again thanks to our relative ignorance, we chose
to proceed with the experiment without knowing that total occlusions would remain one of last hurdles of interventional cardiology, even today.
The right coronary occlusion was initially crossed with a 0.014” wire –a major technological advance at the time– through an 8F guiding catheter. Balloon pre-dilatation was performed at 6 atmospheres with a 3.0/20-mm balloon. The stent had to be hand-crimped onto the balloon prior to deployment. Deployment was performed at 8 atmospheres and post-stenting balloon dilatation was not performed. The patient was pre-treated with 300 mg of aspirin and 75 mg of dipyridamole the day prior to the procedure. At the beginning of the procedure, a continuous infusion of dextran (1000 ml) and a bolus dose of 10,000 U of heparin were administered. Post-procedure, a combination of heparin and oral anticoagulation therapy with coumadin was initiated after sheath removal. The procedure was performed in the afternoon and the patient likely had a better night of sleep than the physicians who performed his procedure, as we could not wait until the morning to bring him back to the cathlab for a re-look. Surprisingly, the vessel was open. This was back in 1987, and you can be sure that if similar circumstances and techniques occurred in 2006, the vessel would be occluded. Better to be lucky, a lesson learned from football. The patient was discharged without complications one week after the index procedure. Despite his above average educational status, the patient decided not to take his medication since he was doing so well with the procedure. Once again this patient was likely correct about his own personal “clinical” decisions because in October, 2000 he underwent angiographic and IVUS follow-up examinations (Figure 1) and had yet to suffer another cardiovascular event.
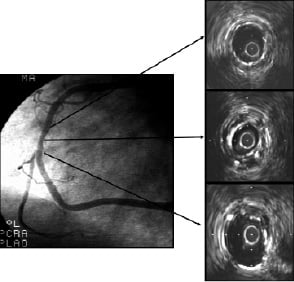
Figure 1. Angiography (right panel) and IVUS (left panels) images 13-years after the first implantation of a Palmaz-Schatz stent in a human coronary artery. IVUS revealed an under expanded coronary stent (middle panel) with almost no intimal proliferation.
There was no significant disease progression in other vessels or any restenosis detected. As you might expect, the stent was underexpanded. The in-stent minimal cross-section area (CSA) was 4.9 mm2, while the mean proximal and distal CSA was 9.02 mm2. The lack of intimal proliferation after the recanalization of a totally occluded vessel 13 years after deployment is puzzling as it resembles the results of the yet to be tested drug-eluting stents. The irony of his destiny was that this patient lived 14 years after the procedure without any cardiovascular complications and, sadly, died after a car accident. Why didn’t he have thrombosis? Coronary disease progression? Restenosis? Other cardiovascular events? The lack of answers to these and many other questions is what makes medicine so inspiring.
The Cypher™ Stent FIM Experience
The need for a device with potent antiproliferative properties soon became apparent4,5. Once again we were contacted by Johnson and Johnson. Their scientists had developed and tested, in various experimental models, a stent coated with sirolimus (rapamycin). The stent was a conventional bare metal stent - laser cut 316L stainless steel balloon-expandable stent coated. The device was coated with a 5 µm thick layer of a non-biodegradable polymer blended with the drug. Rapamycin was originally developed by Wyeth-Ayerst Laboratories and had just been approved by the Food and Drug Administration (FDA) for the prophylaxis of renal transplant rejection at the time of the FIM experience in 1999. Most of the mechanisms of action of the drug were well known, and they were related to the binding of sirolimus, the FKBP complex to a specific cell cycle regulatory protein, the mTOR (mammalian target of rapamycin)6-10. What we did not know was the effect of such potent medication in a diseased human coronary artery. As you might remember, we were just understanding the problems associated with brachytherapy and had poor experiences with previous attempts at stent coatings.
Although we had accumulated an impressive knowledge about the mechanisms of stent deployment, mechanisms of restenosis and how energy and drugs could modulate the cell cycle to prevent proliferation, we were once again walking in the dark, with skepticism at its highest level. Unlike the Palmaz-Schatz FIM experience, which involved the treatment of a single patient, the Cypher FIM required 30 patients. The plan was to perform 15 patients on a single day, so we could appreciate the success or failure of the device at once. Initially IVUS imaging was not proposed, but our group would not miss the opportunity to look at these devices closely and all procedures were guided by IVUS as well as subsequent examinations. Two preparations of sirolimus-eluting stents were tested initially. The fast release formulation (FR) delivers the drug almost completely by 15 days after implantation, while the slow release (SR) formulation, which later became the commercially available Cypher stents, takes > 28 days for complete drug release.
The first session occurred in December, 1999 and the FR platform was selected for the 15 treatments. A month later, after the early safety of SES was confirmed, the SR SES formulations were deployed in another cohort of 15 patients, again in a single day. Yes, our group had improved our deployment techniques and stents were implanted after balloon pre-dilatation and followed by high-pressure (> 12 atmospheres) balloon post-dilatation through a 6F guiding catheter. Yet, the balloons for pre-dilatation and post-dilatation were longer than the stents, as we didn’t yet appreciate the potential risks of a geographical miss at the time. Patients received aspirin (325 mg/day, indefinitely) started at least 12h before the procedure and a 300 mg loading dose of clopidogrel immediately after stent implantation. Dual antiplatelet therapy was maintained for only 2 months.
The 4-month follow-up date of the first group arrived. The Cordis team, at the time led by Judy Jaeger, arrived in Sao Paulo the day before. A mixture of disbelief and optimism increased as each angiogram was being performed. Between cases, Judy provided constant phone feedback to Bob Falotico, who had been involved with the development of this technology since the earliest days. When the angiography of patient number 15 was completed, the group was physically tired of walking up and down the stairs from cathlab room 4 and 7. Angio films and IVUS images were reviewed for each case at the end of the day and the second cohort of 15 patients returned the month following this first excitement. The scene repeated itself. At the end of the day, a group of tired physicians gathered to evaluate and celebrate what would become the first successful experience with drug-eluting stents and likely change the way we would face coronary disease for years to come7-10. The difference this second time was that Dr. Amanda Sousa arranged to have champagne for the celebration.
Patrick Serruys and his team were responsible for the IVUS analysis of the data. Accustomed to evaluate bare metal stents, soon after they received our follow-up IVUS tapes, they sent a request to re-submit the follow-up images because we had “wrongly sent duplicates of the index procedure”. They could not believe that they were indeed looking at correct follow-up images, which were identical to the post-procedure results because the drug had abolished intimal proliferation all together. This was clarified in a meeting at the EuroPCR, when we had the opportunity to show each of the 30 cases with the index and follow-up images side by side.
Unlike the Palmaz-Schatz stent experience described above, the Cypher FIM human testing was much more regulated and closely monitored. A body of pre-clinical experience was available and yet, so little was known. It took the imagination, scientific knowledge and courage of various individuals –including the patients– to embark upon this journey. Paraphrasing Albert Einstein: “If we knew what it was we were doing, it would not be called research, would it?”

