Abstract
“No option” patients are a sizeable cohort of population with refractory angina due to coronary artery disease that is considered unacceptable for revascularisation intervention. Those patients experienced severe angina defined as Canadian Cardiovascular Society angina class III/IV, which persist despite maximal tolerated medical therapy. As multiple factors may affect the perception of pain, the severity of angina symptoms may eventually vary. Over the last decades, hundreds of “no option” patients with refractory angina were enrolled in multiple angiogenesis studies. Patients enrolled in the control arms of those studies may present a unique cohort of “no option” patients in whom persistence of angina could be evaluated. In performing such an analysis, it is noteworthy that patients who received placebo treatment demonstrated significant symptomatic improvement, while those who were enrolled in open label studies and received no treatment experienced improvement very infrequently. We analysed the outcome and changes in angina status among 43 patients who were screened for – but were excluded from – gene therapy phase I and II studies. Mean follow-up was 5.3±2.6 months. Over this period, the combined rate of death, myocardial infarction and revascularisation was 28%. Angina class among the 33 survivors who did not undergo revascularisation did not change over time (2.9±0.5 vs. 2.7±1.2, p=0.8) although inter-patient variability was noted. Collectively the data suggest unfavourable outcome of “no option” patients with refractory angina. While persistence of angina may be present for cohort of patients, spontaneous individual patient fluctuation up to 2 CCS classes may not be infrequent.
Introduction
The quest for effective regenerative strategy to improve myocardial perfusion and function has been translated, over the last decade, into the experimental clinical arena. A conservative estimate is that 5-10% of patients refered to coronary angiography may suffer from advanced symptomatic disease not amenable for contemporary coronary revascularisation1-3. Considering that despite advancements in preventive and interventional cardiovascular medicine, the prevalence of coronary artery disease (CAD), as reported by the American Heart Association was 6.9% (13.5 millions), the number of symptomatic patients with limited revascularisation options is sizeable4. It is important to recognise, however, that the term “no option” ischaemic cardiac patients is a relative one5. The ability to explore innovative therapeutic solutions directed toward complex coronary patients may vary between countries and institutions due to divergence of expertise and the availability of advanced technologies. Thus, in the current concise review, we will refer to “no option” patients as to those who have had advanced symptomatic coronary artery disease not amenable to coronary revascularisation per each of the mentioned study protocols. The term “refractory angina” will be referred to as angina not controlled by revascularisation and medical therapy in patients with objective evidences of myocardial ischaemia, as was suggested by the Joint Study Group on refractory angina3. Finally, as most studies of “no option” patients with refractory angina eventually included individuals with severe angina (or angina equivalent) defined as Canadian Cardiovascular Society (CCS) class III or IV, the current discussion will be focused on those patients.
Assessment of the persistence of refractory angina is challenging. Insights may be derived by evaluating changes in angina status among patients enrolled into multiple angiogenesis studies. These patients include individuals who were randomised to the control arms of double-blind (with the caveat of confounding placebo effect) and open label studies. A third group of patients was composed of those individuals who were referred to, but were excluded from, angiogenesis studies due to non-angina related parameters. Assessment of the persistence of angina among the latter cohort may reflect the “real world” of cardiology practice, manifesting the natural course and prognosis of “no option” refractory angina.
The complexity of “no option” patient definition
Published criteria for “no option” patients varied between studies6-15. There are two major parameters that may affect the status of “no (revascularisation) option”. The first is technological advancements that with time allow intervening in complex lesions that previously considered being either associated with unacceptable risk and/or incapable to provide a meaningful clinical improvement6. For example, incessant restenosis, once a major “no option” inclusion criteria, is now a treatable disease. Similarly, a safer interventional approaches to degenerative saphenous vein graft using a wide range of distal embolic protection devices, aspiration devices and potentially new generations of stent grafts, make this “no option” inclusion criteria obsolete. The second, is progression of atherosclerosis among those patients who already suffer from severe coronary artery disease. Such progression may lead to the development of new coronary lesions that may aggravate angina and thus become new targets for effective intervention even among patients who a priori had no revascularisation options. In one of our early myocardial cell-delivery studies, seven patients sustaining refractory myocardial ischaemia during enrolment underwent subsequent coronary revascularisation over one year follow-up6. Several of the interventions were feasible with the introduction of drug eluting stents, which allowed durable treatment for diffuse coronary disease. Additional patients from the same study underwent coronary revascularisation following 4.5 years due to progression of disease in native coronary artery distal to a graft anastomosis (Figure 1). Thus, the term “no option patient” may be considered as “provisional” and should be limited to the time of patient evaluation.
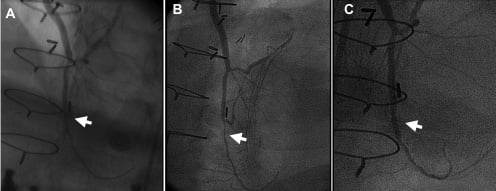
Figure 1. Case example: Serial angiographic views of left anterior descending artery distal to anastomotic site. A. Baseline coronary angiography performed before enrolling into cell therapy study. B. Angiography performed 4.5 years after enrolment due to new onset of angina, demonstrating progression of disease with severe new stenosis (arrow). C. After stent implantation.
Persistence of angina in double-blind, randomised angiogenesis studies
The severity of angina symptoms may be affected by physiological, metabolic and psychological mechanisms, and in many cases are disproportional to the magnitude of myocardial ischaemia. In order to assure inclusion of patients with refractory angina, candidates for angiogenesis studies need to be on maximal tolerated medical treatment prior to study enrolment in order to avoid any change in the background treatment (except for nitroglycerin consumption) throughout the study follow-up6,7.
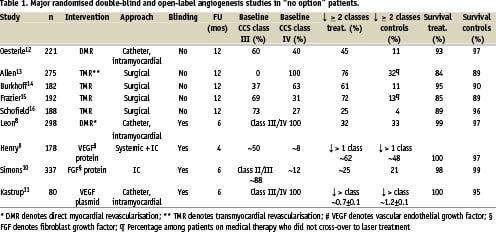
Improvement in angina status was consistently observed among patients enrolled in the control arm of double-blind randomised studies8-11. The magnitude of improvement was similar to those noted in the treated arm, and was related to a potent placebo effect on the one hand, and lack of a clinically relevant treatment effect on the other hand (Table 1). For example, in a recent study of percutaneous laser myocardial revascularisation, 298 patients were randomised in a 2:1 fashion to receive transendocardial laser channels (low- and high-dose), or electromechanical mapping without direct myocardial revascularisation8. Per protocol, all patients had to have severe angina pectoris despite optimal medical therapy and considered to have coronary artery disease unacceptable to revascularisation. Objective evidences for ischaemia included exercise treadmill test and pharmacological, dual isotope perfusion imaging study. CCS angina class improved equally in all groups with an average reduction in the frequency of class III-IV angina of 60% at 6 months, which was maintained at 12 month follow-up. Reduction in angina symptoms was in concordance with assessment of quality-of-life and exercise treadmill parameters including exercise duration and time to 1 mm ST depression. Of note, the adenosine perfusion imaging, an objective measure of ischaemia, did not change over time in any of the three groups. Similarly, in the Vascular Endothelial Growth Factor in Ischaemia for Vascular Angiogenesis (VIVA) study, 178 patients were randomised to low-dose, high-dose and placebo in a double-blind fashion (Table 1)9. There was a consistent improvement among the placebo treated patients both in CCS class and exercise stress tests parameters. Interestingly, CCS class improvement in the placebo group tended to be higher at 2, compared to 4 months.
A potential limitation of the above and other studies is the relatively short term follow-up. Although it is conceivable to assume that placebo effect will decay over time, a recent analysis of angiogenesis studies suggested that placebo effect may be sustained for 3 years17. Interestingly, in analysis of 52 randomised studies comparing placebo treatment with no treatment, the only persistence of placebo effect was noted in studies assessing perception of pain as an endpoint18. Thus, angina improvement following placebo treatment may endure and the true persistence of angina among “no option” patients enrolled in double-blind, randomised, placebo-controlled studies may be underestimated.
Persistence of angina in open-label, randomised angiogenesis studies
Assessment of angina among patients, randomised to the control arm in a non-blinded fashion, may provide a more realistic reflection of temporal changes in angina severity. During the last decade, several catheter based and surgical laser based myocardial revascularisation studies were conducted (Table 1)12-16. In those studies, patients enrolled in the control arm underwent no sham procedures, and therefore were not subjected to placebo treatment. Indirect comparison of changes in angina severity among those patients compared to patients enrolled in control arms in double-blind studies suggest that the magnitude of improvement among non-treated compared to placebo-treated patients is lower with an average of 20% (4-32%) (Table 1). In addition, since up to one third of the medically treated patients in the open-label studies crossed over to laser treatment, the frequency of significant improvement in angina is probably lower. Thus, it is conceivable to assume that over a 12 months follow-up, the vast majority of patients with CCS class III/IV refractory angina who had no intervention experienced no significant improvement. Milder improvement of < 1 CCS class, however, cannot be excluded.
Refractory angina among candidates excluded from angiogenesis studies
Candidate for therapeutic angiogenesis studies who were excluded due to none angina related status may represent the “real world” population of “no option” patients with refractory angina. Few data exist to explore the clinical course and outcomes of these patients. Accordingly, we have used our database in order to evaluate the angina severity and clinical outcomes of patients who were excluded from phase I and II gene therapy studies that have been conducted in our institutions.
Study design
Patients who were excluded from phase I and phase II randomised, double-blind, placebo controlled studies of transendocardial delivery of adenovirus encoding for vascular endothelial growth factor 121 isoform (AdVEGF121) were evaluated for clinical outcomes. Patients were eligible for the study if they were between the ages of 18-80 years old, had severe or critical stable angina pectoris (CCS Angina Class III or IV) despite maximal medical therapy and had no coronary artery revascularisation alternatives7. Exclusion criteria were recorded and specified (see below).
Results
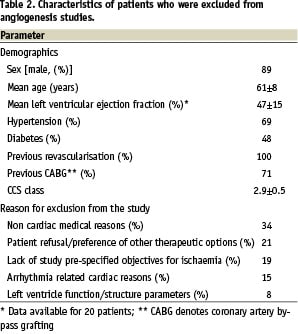
The study cohort consisted of 43 patients – 27 were screened for phase I and 16 were screened for the phase II study. Patient characteristics and reasons for exclusion are listed in Table 2. Main non-cardiac exclusion reasons included retinopathy, hepatitis and renal failure; main cardiac related reasons included chronic atrial fibrillation, presence of pacemaker and/or defibrillator, reduced left ventricular function or presence of thin myocardial wall at the target region. The latter criteria was due to intra-myocardial injection mode of delivery that mandated a viable, thick myocardium (i.e. > 8 mm end-diastolic thickness) in order to instil the experimental compound into the myocardium. Interestingly, 19% of the patients, despite having CCS class III, did not have objective evidence of ischaemia using either exercise and/or nuclear imaging at the study pre-specified threshold and thus were excluded.
Mean follow-up was 5.3±2.6 months. Over this period, 3 (7%) patients died, 2 (4.5%) experienced myocardial infarction, and 7 (16%) patients underwent revascularisation (3 underwent CABG and 4 had percutaneous revascularisation). The combined rate of death, myocardial infarction and revascularisation was 28%. Revascularisation became optional shortly after screening in 4 patients and during late hospitalisation for rest angina, in additional 3 patients. Interestingly, all patients who underwent revascularisation in early stages had substantial symptomatic improvement, while none of those with late interventions experienced any improvement in angina. When patients with early revascularisation were excluded from the analysis, the rate of revascularisation interventions and major adverse cardiac events were 7% and 19%, respectively. Angina class among the 33 survivors who did not undergo revascularisation did not change over time (2.9±0.5 vs. 2.7±1.2, p=0.8). Overall 8 patients experience improvement in angina class (1.7±0.9), 9 had no change and 12 experienced worsening of angina. In 4 patients CCS class was not evaluated.
Conclusions
According to our aforementioned data, the overall experience with “no option” patients screened for, but excluded from angiogenesis studies, suggest unfavourable outcome. Of note, although the average angina severity among the studied cohort did not change over the 6 month period, individual patient-based analysis suggests spontaneous changes of up to 2 CCS classes. Thus, the chosen cut-off for significant improvement of > 2 classes, as was reported in some, but not all of the angiogenesis studies, is of clinical relevance.
Summary
Assessment of time-dependent changes in severity of angina among “no option” patients is complex. Multiple factors including spontaneous fluctuations in symptoms, availability of new technologies, which allow advanced new revascularisation intervention along with progression of coronary artery disease affect the persistence of symptoms. Analyses of data derived from angiogenesis studies in “no option” patients suggest strong placebo effect. Recent data from a cohort of “no option” patients who were excluded from similar studies suggest overall unfavourable outcome, albeit with wide inter-patient variability in the persistence of angina. Studies designed for patients with refractory angina may need to include multiple time points for assessment of angina, to exclude patients who undergo additional interventions during follow-up and to predefine significant improvement in angina class as reduction of > 2 CCS classes. Finally, the optimal modality to assess efficacy in terms of myocardial perfusion and function has varied among studies and is yet to be determined.

