Introduction
Heart failure is one of the major unsolved problems in cardiology and one of the most prevalent causes of death. The most important cause of heart failure is coronary heart disease. Within a few years after a myocardial infarction, approximately 20-50% of the patients develop heart failure1,2. Medical and surgical treatment both have major limitations.
Heart failure patients with clinical manifestation as assessed by the ACC/AHA, NYHA classification show a two-year-mortality rate for class I patients of 10%, class II patients of 20%, class III patients of 30 to 40% and class IV patients a two-year-mortality rate of up to 50%.
Heart failure is more common in patients with an anterior infarction3. This is due to the amount of myocardium affected and also due to the curved geometry and thinner nature of the apex. The associated remodelling and wall stress produce an inefficient and often failing left ventricle. Fifty seven percent of patients with an anterior infarction develop heart failure one year after thrombolytic therapy for an acute MI4.
Heart failure after myocardial infarction (MI) is a progressive disease5. There have been many advances in medical treatment the last two decades with the addition of beta-blockers, ACE-inhibitors and angiotensin-receptor-blockers but the incidence of morbidity and mortality for post-MI patients continues to be excessive6,7. However, these regimens have certain limitations, especially when a left ventricular antero-apical aneurysm is present. Surgical aneurysm resection has evolved over the last 50 years. It has included closure of the left ventricle either directly (linear closure first reported by Cooley8), by implantation of a patch or by using the Batista method9. However, favourable outcomes have only been reported in selected patients because of high morbidity and mortality rates10.
A novel implantable device has been developed to treat patients with left ventricular dysfunction and apical wall motion abnormalities. It may deter progressive ventricular remodelling by partially isolating the dysfunctional region. Isolation is achieved by implanting an umbrella-like membrane, the Ventricular Partitioning Device (VPD) within the left ventricle apex. The hypothesis is to decrease both chamber volumes and myocardial stress while improving haemodynamics. (Figures 1 a and b)
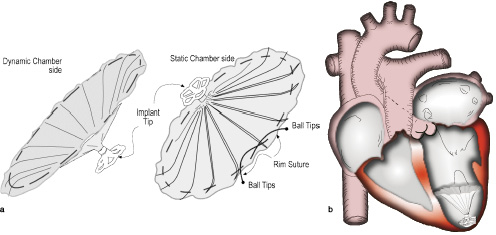
Figure 1. a. VPD device: An ePTFE membrane bonded to an expanded Nitinol frame in the configuration of an inverted umbrella. Currently available in two sizes 85 and 75 mm. b. Implant positioned in left ventricular apex.
This report features our first implantation of this new implantable device in a 70-year-old male patient. The procedure was successful without complications. His one month follow-up revealed his clinical status to be stable.
Case report
A 70-year-old male patient (176 cm, 70 kgs) sustained an anterior myocardial infarction in 1998 followed by coronary bypass surgery in 1999.
Clinically, the patient had refractory class III NYHA heart failure despite aggressive medical treatment. Surgical left ventricle resection was recommended but refused by the patient.
ECG showed sinus rhythm and a right bundle branch block with deep Q-waves (V1 to V6), elevated St-segments in V1 to V4 and terminal T-depression.
Echocardiograms demonstrated a dilated left ventricle (60 mm) and left atrium (55 mm) with moderate reduced left ventricular function. No apical thrombus was found. Mild aortic stenosis, moderate aortic regurgitation and moderate mitral regurgitation were also present. Pulmonary artery pressure was normal.
Blood laboratory was normal, except BNP 8 times elevated over normal range. The patient was treated with standard doses of aspirin, ramipril, furosemid molsidomin, isosorbidnitrate and carvedilol.
The implantation was performed using local anaesthesia. A 14 F sheath was placed using femoral arterial access.
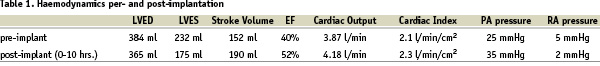
Potential active coronary ischaemia was ruled out by a recent coronary angiography. Pre-implantation a right heart catheterization was performed via the left femoral vein revealing a pulmonary artery systolic pressure of 25 mmHg and a mean pulmonary wedge pressure of 5 mmHg. A 7 Fr pigtail was advanced via the left femoral artery into the left ventricle to obtain pressure recordings and left ventriculography. The ejection fraction was 40%. Calculated cardiac output was 3.87 L/min (cardiac index: 2 L/min/m2).
To determine the device size, the cross-section of the ventricular chamber was measured three and a half cm proximal to the apex on initial ventriculogram. For an apical length of 62 mm and a diameter of 40 mm, we found the 85 mm device to be appropriate (an oversizing of 30 to 50 % for implantation is recommended).
The umbrella-like implant was delivered via an access system that compromised a guiding catheter and a delivery system using Amplatz Extra Stiff .035, 260cm long guidewire.
The device preparation involved collapsing the implant after attaching it to the delivery catheter.
The guide catheter is a braided catheter with a preformed distal tip that is designed for orientation into the left ventricular apex. The guide catheter with the dilator was advanced retrogradely across the aortic valve and placed in the apex of the left ventricle. A left ventriculogram was performed using the side port of the guiding catheter to visualize the landing zone. Once in place, the delivery catheter was used to advance the VPD implant within the guide. The device was easily released using a distal screw mechanism. To expand the device, a compliant balloon located proximal to the screw connector was inflated after being advanced beyond the guide catheter during deployment in the left ventricle. This allowed device anchoring with an expansile foot against the endocardium. (Figures 2 a and b)
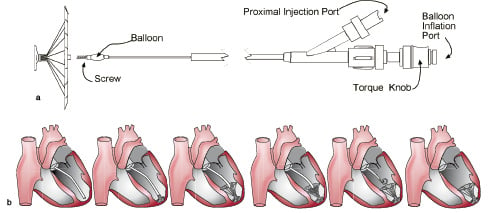
Figure 2. a. Delivery catheter and implant. b. Placement of the VPD implant device in the left ventricle.
The balloon was deflated, the delivery device disengaged and removed from the guide catheter. A final angiogram showed a trace leak at the side of the ventricular region where the device was implanted.
Total procedure time was 140 minutes and fluoroscopy time 17.2 minutes. Ejection fraction immediately post-implantation was increased from 40 to 52% due to the altered left ventricular geometry and diastolic volume (Figures 3 a and b).
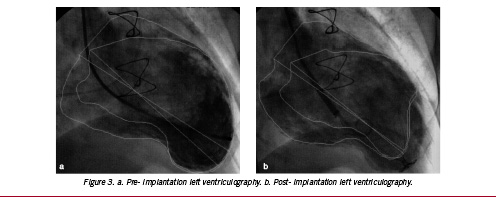
Figure 3. a. Pre- implantation left ventriculography. b. Post- implantation left ventriculography.
Stroke volume increased from 152 ml to 190 ml. Ten hours following the implant procedure a right heart catheterization recorded a pulmonary artery systolic pressure 35 mmHg and cardiac output had increased from 3.87 to 4.18 L/ min as shown in Table 1.
The patient was discharged home without complications two days following the intervention on aspirin (100 mg) and clopidogrel (75 mg) in addition to his previous medication.
There were neither peri- nor post-procedural complications (up to one month following the implantation). His one month clinical follow-up revealed his situation to be stable.
Discussion
The device implant was easily implanted without complication. Improvement in left ventricular function was noted after deployment of the device as measured by increases in cardiac output, stroke volume and ejection fraction and a decrease in end-diastolic and -systolic volume.
Long term follow-up will clarify the implant´s potential clinical significance.
This data confirms that the VPD Implant is able to partition an enlarged left ventricle with an apical wall motion abnormality into dynamic and static chambers. The static chamber is the apical portion of the left ventricular volume that is assumed to seal, removing it from circulation. The segregated blood in the static chamber may also form organized thrombus behind the partitioning membrane, eliminating embolic potential. Wall stress in the partitioned myocardium may decrease, eliminating the stresses responsible for dilation.
In addition to the potential regional unloading, the reduction in size of the dynamic chamber may result in a decrease of the myocardial stress in the normal myocardium via Laplace´s Law, providing a global unloading of the ventricle.
The assumed combined effect of regional and global unloading may have important consequences for myocardial energetics. Regional unloading may decrease the left ventricle´s myocardial oxygen demand ratio. Future studies will be needed to better answer these questions.

