Abstract
The percutaneous treatment of structural, valvular, and non-valvular heart disease (SHD) is rapidly evolving. The Core Curriculum (CC) proposed by the EAPCI describes the knowledge, skills, and attitudes that define competency levels required by newly trained SHD interventional cardiologists (IC) and provides guidance for training centres. SHD ICs are cardiologists who have received complete interventional cardiology training. They are multidisciplinary team specialists who manage adult SHD patients from diagnosis to follow-up and perform percutaneous procedures in this area. They are competent in interpreting advanced imaging techniques and master planning software. The SHD ICs are expected to be proficient in the aortic, mitral, and tricuspid areas. They may have selective skills in either the aortic area or mitral/tricuspid areas. In this case, they must still have common transversal competencies in the aortic, mitral, and tricuspid areas. Additional SHD domain competencies are optional. Completing dedicated SHD training, aiming for full aortic, mitral, and tricuspid competencies, requires at least 18 months. For full training in the aortic area, with basic competencies in mitral/tricuspid areas, the training can be reduced to 1 year. The same is true for training in the mitral/tricuspid area, with competencies in the aortic area. The SHD IC CC promotes excellence and homogeneous training across Europe and is the cornerstone of future certifications and patient protection. It may be a reference for future CC for national associations and other SHD specialities, including imaging and cardiac surgery.
The treatment of structural, valvular, and non-valvular heart disease (SHD) has been revolutionised by the emergence and development of percutaneous techniques. Percutaneous treatment of adult SHD is now a mature field supported by robust scientific evidence developed in recent decades and has been associated with significant benefits for both individuals and populations. While it is expected by both regulation authorities and patients that physicians will have had guidance for individual training (cardiologists, imaging cardiologists, cardiac surgeons, and first use of interventional cardiologist) in managing patients suffering from SHD, such recommendations are still lacking. The current Core Curriculum (CC) for percutaneous SHD interventions has been designed by the European Association of Percutaneous Cardiovascular Interventions (EAPCI) as the first key step to filling this gap in interventional cardiology. This innovative document has been prepared in collaboration with the European Association of Cardiovascular Imaging (EACVI) and the Cardiovascular Surgery Working Group (WG CVS) of the European Society of Cardiology (ESC). It will stimulate future Core Curricula in other SHD specialities where EAPCI is available to offer support and expects to collaborate, including imaging and cardiac surgery.
This curriculum was organised to follow and complement the Core Curriculum for Percutaneous Cardiovascular Interventions (2020) by the EAPCI Training and Certification Committee (TCC)1.
Introduction
The present SHD CC aims to support the educational requirements of an updated European consensus. It defines and standardises competency levels required for percutaneous SHD interventions to treat patients with heart failure and cardiac symptoms related to valve disease or to prevent complications of thromboembolic diseases (Figure 1).
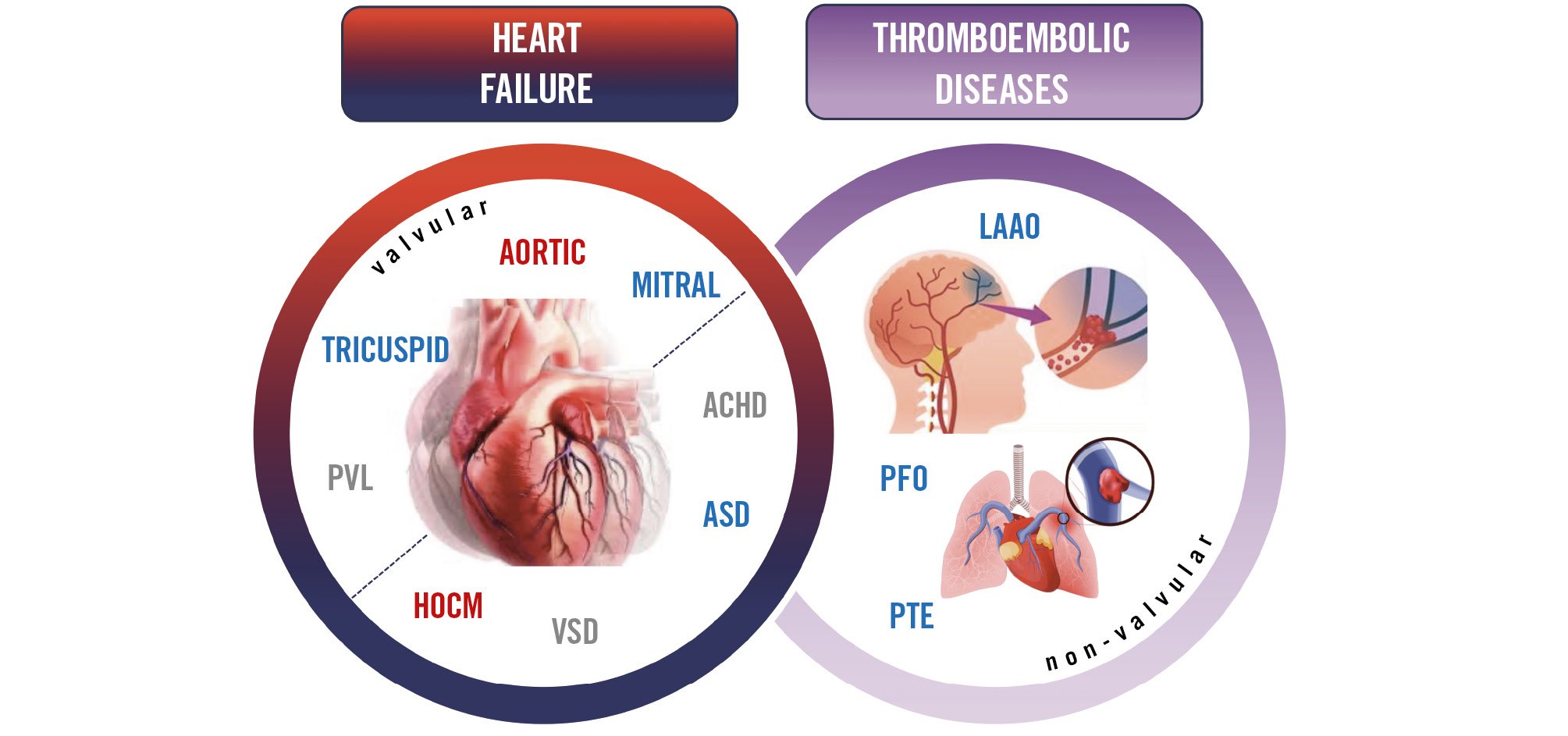
Figure 1. The spectrum of cardiology areas treated by percutaneous structural heart disease interventions: valvular (aortic valve, mitral valve, tricuspid valves, paravalvular regurgitation) and non-valvular (hypertrophic obstructive cardiomyopathy septal ablation, ventricular septal defect, atrial septal defect, adult congenital heart disease, patent foramen ovale, left atrial appendage occlusion and pulmonary thromboembolism). These interventions can be used separately – or combined – to treat patients with thromboembolic diseases or heart failure. The colour code is consistent with the prevailing percutaneous treatment route, arterial (red), venous (blue) or mixed (red/blue). ACHD: adult congenital heart disease; ASD: atrial septal defect; HOCM: hypertrophic obstructive cardiomyopathy; LAAO: left atrial appendage occlusion; PFO: patent foramen ovale; PTE: pulmonary thromboembolism; PVL: paravalvular regurgitation; VSD: ventricular septal defect
Methodology
The writing task force included members with substantial expertise in different aspects of SHD percutaneous interventions nominated by EAPCI. Detailed data on this document’s preparation process are found in the “2023 EAPCI Core Curriculum for Percutaneous Structural Heart Disease Interventions Extended Version” and are available upon request to the corresponding authors (Figure 2).
The 2023 EAPCI Core Curriculum for Percutaneous Structural Heart Disease Interventions Extended Version includes a comprehensive description of the specific components in 5 areas, organised into 114 chapters and subchapters. Each section includes statements on the objectives and is further subdivided into the required objectives, knowledge, skills, behaviours, ESC topic list, essential reading, and attitudes.
This manuscript aims to be a reference for future iterations that will occur under the auspices of the EAPCI (Central illustration).
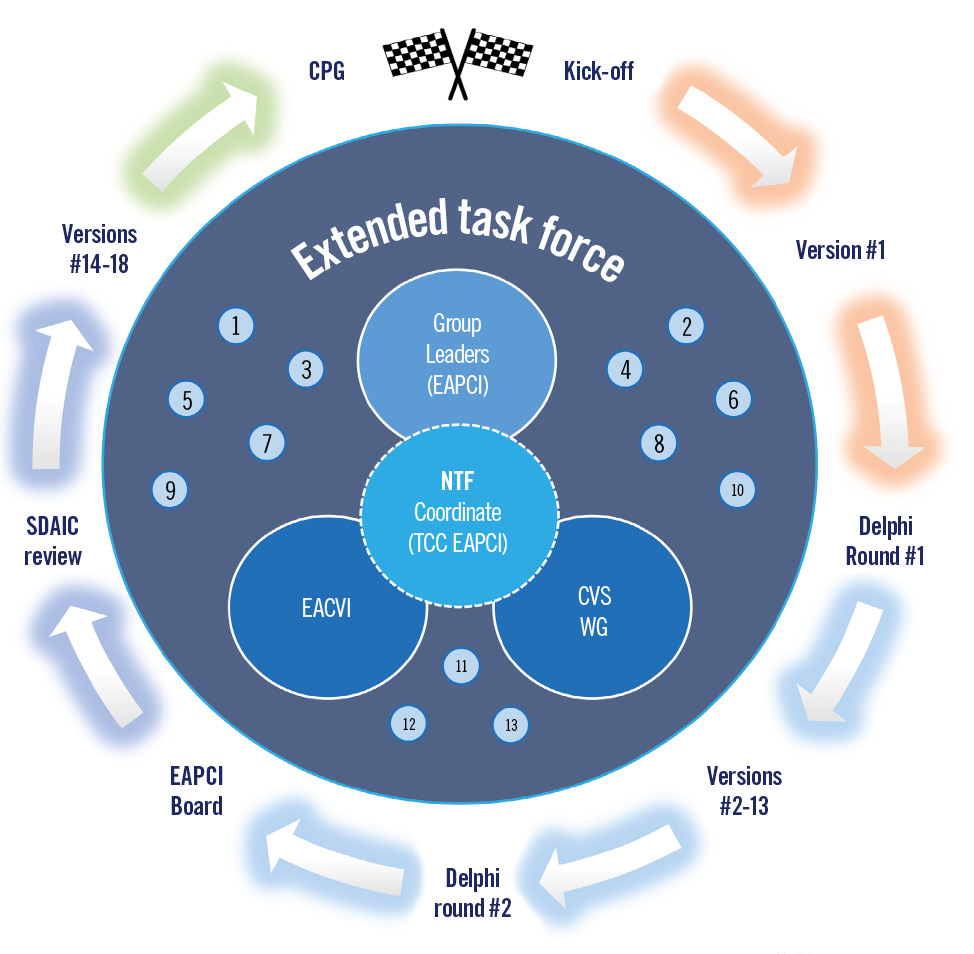
Figure 2. Methodology of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Training and Certification Committee (TCC) Task Force (TF): the writing TF included three coordinating authors, a Nuclear Task Force (NTF) of 13 lead authors & group coordinators, and an Extended Task Force of 34 members, including the European Association of Cardiovascular Imaging (EACVI) and the Cardiovascular Surgery Working Group (WG CVS) of the European Society of Cardiology. The document was blindly revised by the EAPCI Scientific Documents and Initiatives Committee (SDAIC) and concluded after revision by the NTF and EAPCI Board. This version was circulated, revised, and approved by all authors. Finally, the Clinical Practice Guidelines (CPG) Committee formally approved the final version.
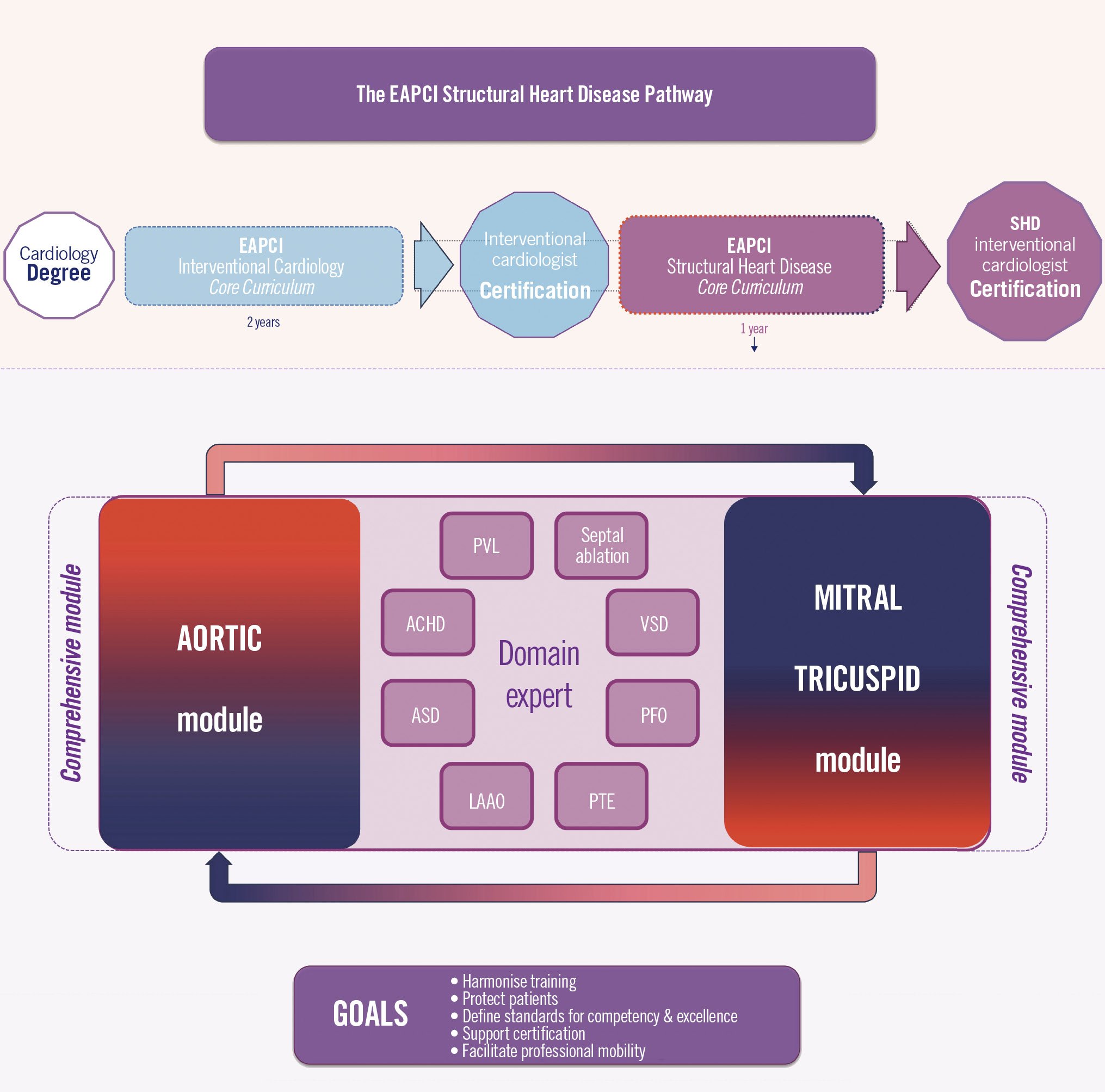
Central illustration. The EAPCI Structural Heart Disease Training and Certification Pathway. Starting with a general cardiology degree, the interventional cardiologist training follows the EAPCI Interventional Cardiology (IC) Core Curriculum to acquire the EAPCI Interventional Cardiology Certification. Sequentially, the same training path follows the EAPCI Core Curriculum for Percutaneous Structural Heart Disease (SHD) Interventions to acquire the SHD Certification. An overlap between IC and SHD-IC certifications is accepted, allowing the IC to be certified 30 months after starting the IC Certification. Within the SHD Certification, the same principle is envisaged. While a comprehensive aortic/mitral/tricuspid is recommended, further dedicated training using the aortic or the mitral/tricuspid module is also possible, allowing SHD training to be completed in 1 year. The additional domains can be acquired in parallel when conditions permit. ACHD: adult congenital heart disease; ASD: atrial septal defect; EAPCI: European Association of Percutaneous Cardiovascular Interventions; LAAO: left atrial appendage occlusion; PFO: patent foramen ovale; PTE: pulmonary thromboembolism; PVL: paravalvular regurgitation; VSD: ventricular septal defect
EAPCI Core Curriculum for Percutaneous Structural Heart Disease Interventions
THE CLINICAL FIELD OF PERCUTANEOUS STRUCTURAL HEART DISEASE INTERVENTIONS
A solid background in coronary interventions, peripheral artery disease, and management of any procedural complications is needed, requiring a level of competence in interventional cardiology that is equal to or above the EAPCI Interventional Cardiology Core Curriculum 202012345.
The SHD CC differentiates between an “SHD IC” and a “Domain expert”: the first chooses a comprehensive training in the Aortic (AOR) and/or Mitral/Tricuspid (MTC) modules. The latter is an IC whose differentiation is limited to particular areas: aortic, mitral, tricuspid, paravalvular regurgitation, septal ablation, adult congenital heart disease, ventricular septal defects, atrial septal defects, patent foramen ovale, left atrial appendage occlusions or pulmonary thromboembolism (Figure 3).
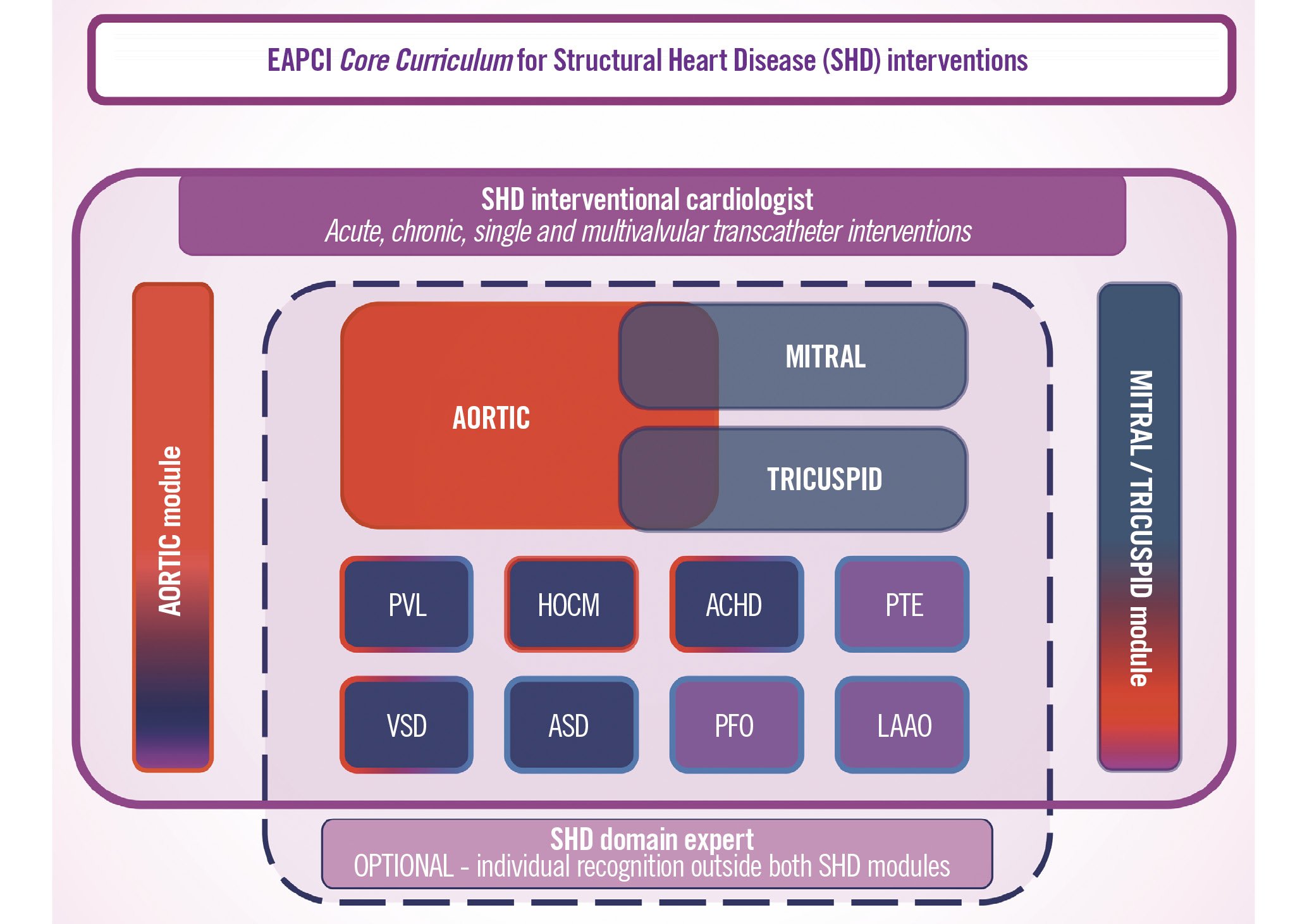
Figure 3. The EAPCI Core Curriculum for Percutaneous SHD Interventions Training plan, modules, and domains: after the cardiology degree (minimum of 4 years of training) and interventional cardiologist (IC) certification (2 years) the trainee can engage in the comprehensive training module, the aortic/mitral/tricuspid (AoMTC), or choose between the aortic (AOR) and mitral/tricuspid (MTC) modules, respectively (red or light blue background, respectively). These modules are complementary, since each trainee involved in one module should still acquire minimal expertise from the other one. The recommended minimum duration of each of the two dedicated SHD IC training modules should be one year of training in SHD IC for each one of these areas, or 18 months when both are acquired in combination (AoMTC). In addition, as part of each module, the trainee will also have to acquire basic expertise in other specific SHD areas where full expertise can be acquired outside (or on top) of each SHD module (Figure 2) (dark blue or magenta backgrounds, that are related, respectively, with prevailing heart failure or thromboembolic disease). This optional “Domain expertise” recognition is designed to acknowledge the differentiation in any particular area included in the EAPCI SHD IC CC, without the requirement for transversal competencies as above: aortic intervention, mitral and tricuspid intervention, paravalvular regurgitation (PVL), septal ablation (HOCM), adult congenital heart disease (ACHD), ventricular septal defect (VSD), atrial septal defect (ASD), patent foramen ovale (PFO), left atrial appendage occlusion (LAAO) and pulmonary thromboembolism (PTE). Within the SHD Certification, practice overlap is possible to allow training in the Aortic, Mitral/Tricuspid modules, and any other domain can be acquired in parallel when conditions permit. The colour code is consistent with the prevailing disease: thromboembolic (magenta) or heart failure (red or blue background), and the competencies are delimited by different lines according to the usual percutaneous treatment route: arterial (red), venous (blue) or mixed (red/blue). SHD: structural heart disease
GENERAL ASPECTS OF TRAINING IN PERCUTANEOUS SHD INTERVENTIONS
The candidates should be cardiologists licensed to practice IC in their country of training. A candidate should have completed a minimum of four years of training in general cardiology and two years of full-time training in IC. As part of the training in interventional cardiology, it is assumed that the IC has achieved all Level IV and Level V competencies described in the EAPCI IC CC1.
The trainee should have had exposure to an appropriate mix of aortic and mitral/tricuspid as well as acute and elective cardiac care, including mandatory, strongly recommended or recommended elements, as described below in Figure 46789.
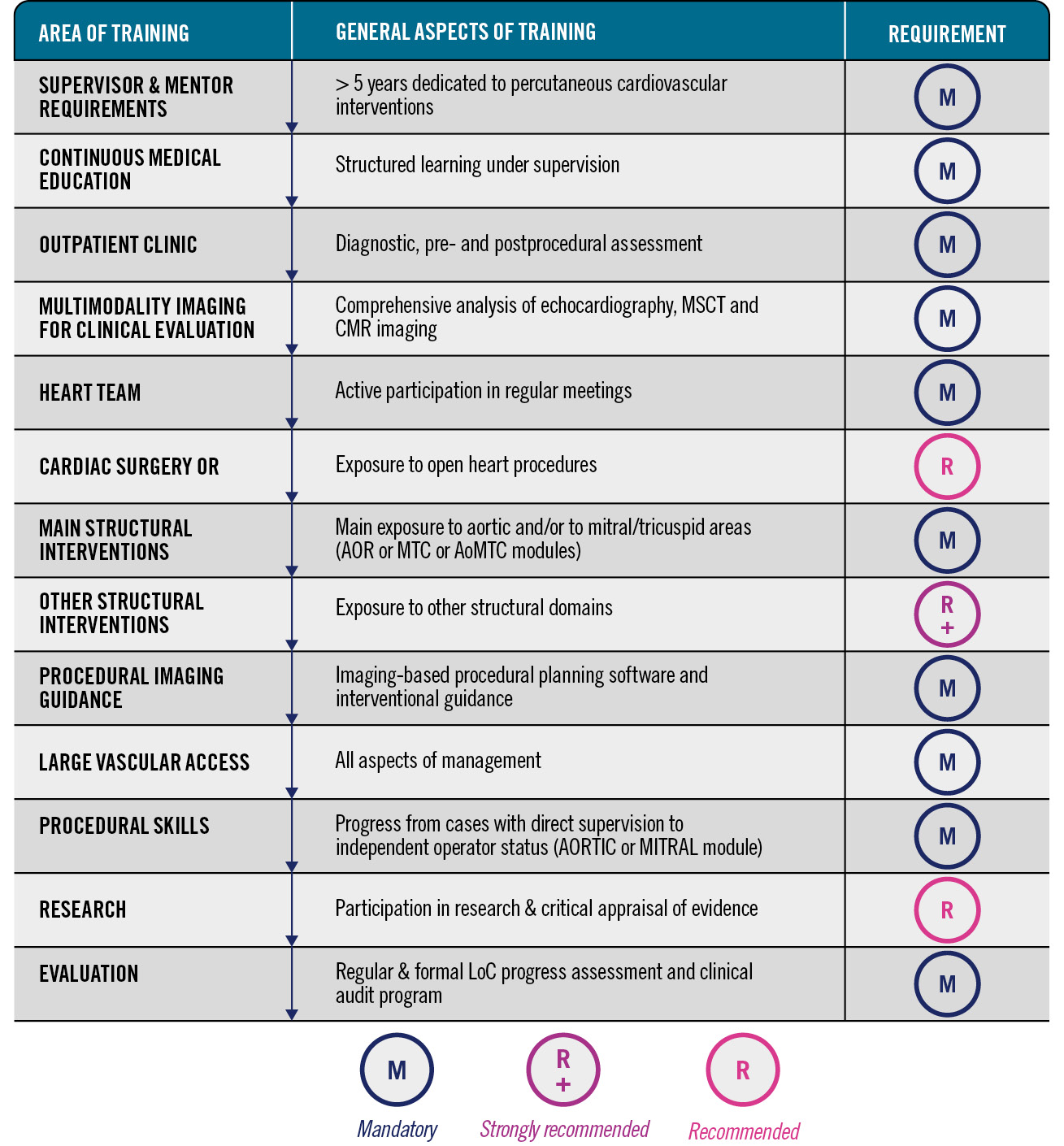
Figure 4. Summary of the structured requirements for percutaneous training programmes in structural heart disease. AOR: aortic; CMR: cardiac magnetic resonance; LoC: level of competence; MSCT: multislice computed tomography; MTC: mitral/tricuspid; OR: operating room
LEARNING OBJECTIVES
The trainee’s education should include the competency domains of interventional cardiology: knowledge, skills, and attitudes which are defined below and should be reinforced during ongoing training. This section describes the definition of EAPCI levels of competence (LoC) recommendations for procedural or non-procedural skills. Their ascending order is summarised in Figure 5.
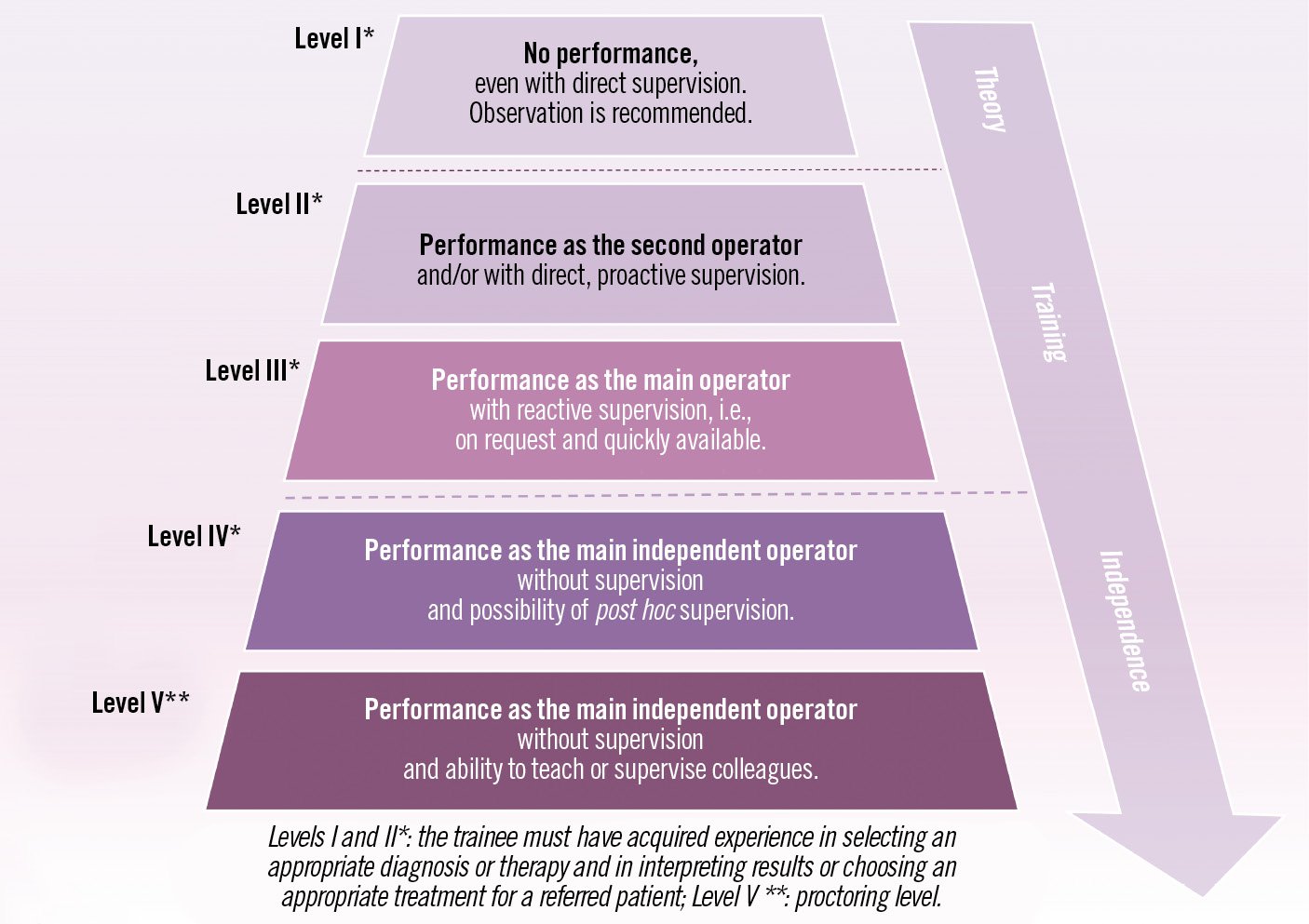
Figure 5. Description of levels of competence (LoC) for non-procedural or procedural skills (adapted from Entrustable Professional Activities).
REQUIREMENTS FOR TRAINING INSTITUTIONS, TRAINEES, AND TRAINERS
A percutaneous SHD training centre is an institution or healthcare network that performs SHD procedures and provides a structured training program for certified interventional cardiologists aiming to achieve the required EAPCI SHD CC LoC in a favourable environment.
The technical portfolio, organisation, referral network, volume and performance of the SHD training centre define the extent and quality of training in SHD10. The institution and affiliates must comply with the requirements and recommendations of their national regulatory bodies first and follow the ESC recommendations and Heart Valve Centres concept, second11. An SHD training centre should have an established clinical, research and SHD training programme121314151617181920.
Trainers should be recognised IC specialists, trained and certified in SHD (where available), and actively involved in the clinical and research activities of the local Heart Team. Their number should always match or exceed the number of trainees. The LoCs that a trainee needs to achieve at the end of his training period are summarised in Figure 6. A more detailed description of each area of training is provided in the extended version of the document. (https://www.escardio.org/Sub-specialty-communities/European-Association-of-Percutaneous-Cardiovascular-Interventions-(EAPCI)).
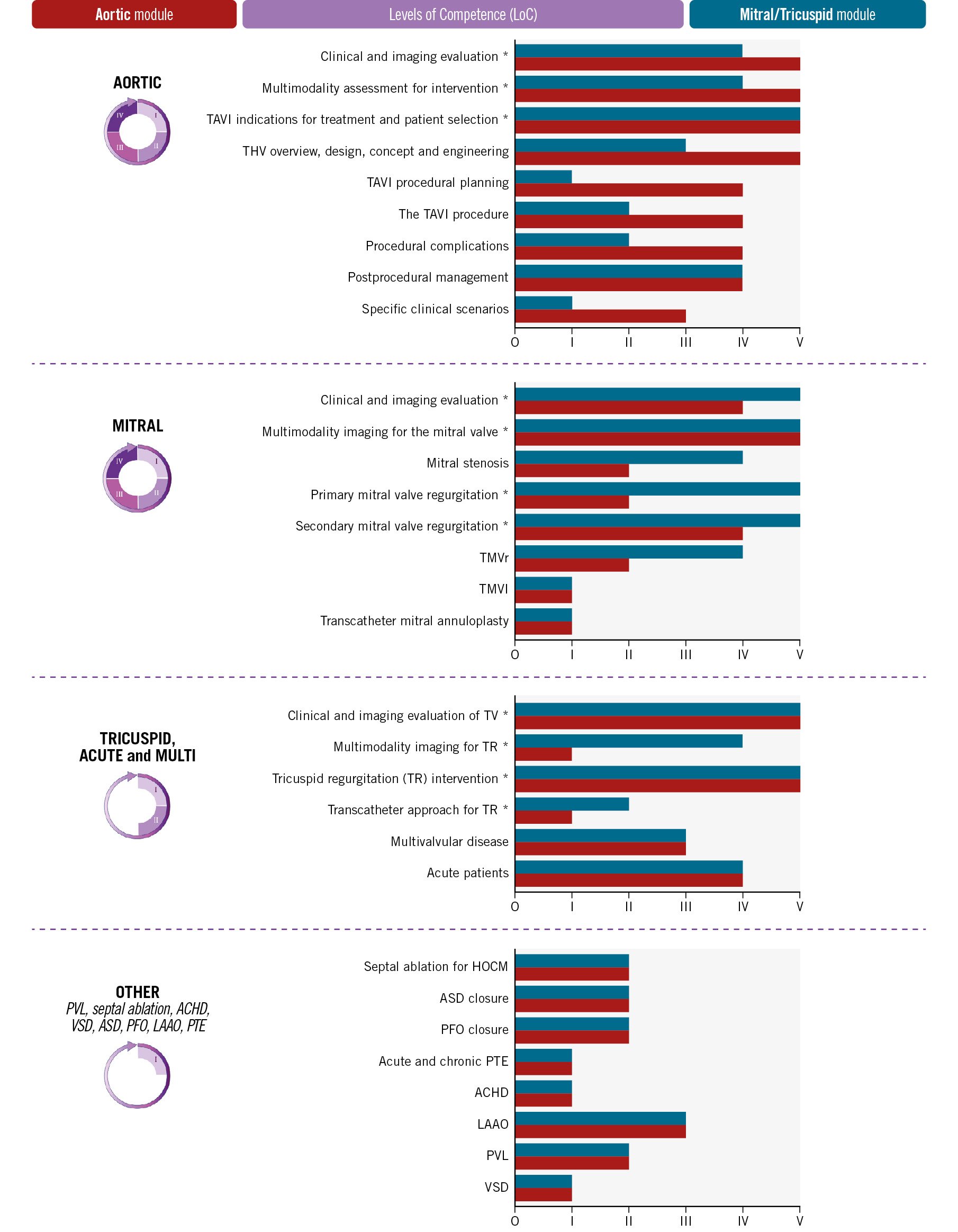
Figure 6. Condensed comparison of the level of competence (LoC) for non-procedural (*) and procedural interventional cardiology skills in the AOR or MTC modules of the SHD IC (from I/1 to V/5). The AoMTC training requires, for any competence, the highest LoC of either AOR or MTC and is not presented to prevent confusion. The optional “Domain expert” recognition demands all theoretical requirements and a minimum LoC IV in all the domains of interventional cardiology, which are defined in their chapter or subchapter: aortic, mitral/tricuspid, septal ablation (HOCM), paravalvular leak (PVL), ventricular septal defect (VSD), atrial septal defect (ASD), adult congenital heart disease (ACHD), patent foramen ovale (PFO), left atrial appendage occlusion (LAAO) and pulmonary thromboembolism (PTE). HOCM: hypertrophic obstructive cardiomyopathy; TAVI: transcatheter aortic valve intervention; THV: transcather heart valve; TMVR: transcatheter mitral valve replacement; TMVI: transcatheter mitral valve intervention; TV: transcatheter valves
Conclusions
The Percutaneous Valvular and Structural Heart Disease Interventions Core Curriculum of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) provides guidance for training centres and trainees.
It describes the knowledge, skills, and attitudes that define competency levels required from newly trained interventional cardiologists performing structural heart disease interventions. They should train within multidisciplinary teams, managing adult patients from diagnosis to follow-up, developing selective skills in either aortic and/or mitral/tricuspid areas. Their education may be complemented by competencies in other domains such as adult congenital heart disease, left atrial appendage occlusion, pulmonary thromboembolism, paravalvular regurgitation, septal ablation or septal defects.
The Core Curriculum promotes excellence and universal training in ESC countries, forming the cornerstone of future certifications for patient protection.
Data availability statement
No new data were generated or analysed in support of this document.
Acknowledgements
The Clinical Practice Guidelines (CPG) Committee from the European Society of Cardiology formally approved the document.
Conflict of interest statement
E. Agricola has received speaker honoraria and compensation from GE HealthCare, outside the current topic. I.J. Amat-Santos has received speaker honoraria and advisory board compensation from Boston Scientific, Meril Life, Medtronic, and Abbott. A. Baumbach has received institutional research support from Biotronik; and honoraria from Faraday, Pi-Cardia and Meril Life. D. Blackman has acted as a consultant, advisory board member, and speaker for Abbott, Edwards Lifesciences, and Medtronic. N. Bonaros has received speaker honoraria from Edwards Lifesciences and Medtronic; as well as research grants from Edwards Lifesciences and Corcym. M. Czerny is consultant to Terumo Aortic, Medtronic, NEOS, and Endospan. O. De Backer has received research grants, speaker and consulting fees from Abbott, Boston Scientific, and Medtronic. P. Deharo has received honoraria from Boston Scientific, Abbott, Asahi, Medtronic and Novartis. P. Lurz has received institutional fees and research grants from Abbott, Edwards Lifesciences, and ReCor; honoraria from Edwards Lifesciences, Abbott, Innoventric, ReCor and Boehringer Ingelheim; and has stock options with Innoventric. R. Hermanides has received compensation from companies outside the current topic. S. James has received institutional research support from Edwards Lifesciences and Medtronic; and proctoring fees from Medtronic. F.R. Joshi has received honoraria and advisory board compensation from Boston Scientific; and travel support from Millbrook Medical. P. Kala declares that he has received consultant and speaker fees from Boston Scientific, Edwards Lifesciences, Sanofi, Novartis, and Servier; participated in advisory boards from Boston Scientific, Abbott, Novartis, and Servier; and received research support from Bayer, Novartis, and Amgen. N. Karam has received consultant fees from Abbott, Medtronic, Edwards Lifesciences, and Boston Scientific. A. Luz has received consultant fees from Abbott. J. Mehilli has received speaker honoraria and compensation from AstraZeneca, Boston Scientific, Daiichi Sankyo, and Shockwave. D. Mylotte has received research grants from Boston Scientific; and speaker honoraria/advisory board compensation from Medtronic, Microport, and Boston Scientific. R. Nuis has received research grant support from Vifor Pharma; and consulting fees from Edwards Lifesciences, Abbott, and Boston Scientific. V. Paradies declares research grants from Abbott to the institution; and speaker fees from Abbott and Boston Scientific. R. Parma has received speaker fees from Edwards Lifesciences. A. Rück declares institutional research and educational grants from Boston Scientific and Edwards Lifesciences; and personal speaker and consultancy fees from Boston Scientific, Abbott, Edwards Lifesciences, and Anteris. T. Pilgrim reports research, travel or educational grants to the institution without personal remuneration from Biotronik, Boston Scientific, and Edwards Lifesciences; and speaker fees and consultancy fees to the institution from Biotronik, Boston Scientific, Edwards Lifesciences, Abbott, Medtronic, Biosensors, and Highlife. G. Tarantini has received speaker honoraria/advisory board compensation from Edwards Lifesciences, Boston Scientific, Medtronic, Abbott, Philips, and Microport. D. Tchétché is consultant for Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic. A. Uebing has received advisory board compensation from Medtronic. N. Van Mieghem has received research grant support from Abbott, Boston Scientific, Medtronic, AstraZeneca, and Daiichi Sankyo; and scientific advisory fees from Anteris, JenaValve, Amgen, Siemens, Pie Medical, Abbott, Boston Scientific, Medtronic, AstraZeneca, and Daiichi Sankyo. M. van Wely has received proctoring fees from Abbott; and speaker fees from Boston Scientific. V. Veulemans has received consulting fees, travel expenses, or study honoraria from Medtronic, Edwards Lifesciences, and Boston Scientific. The other authors have no conflicts of interest to declare.

