Abstract
Aims: A percutaneous mitral annuloplasty device for implantation in the coronary sinus was evaluated in surviving sheep with ischaemia-induced mitral regurgitation.
Methods and results: Microspheres were injected in the left coronary artery of thirty-seven sheep. The treatment was repeated in one month intervals until the resulting myocardial infarctions caused significant mitral regurgitation. Fourteen animals developed a mitral regurgitation degree 2 or higher after 130±16 days and 3.4±0.4 treatments. The annuloplasty device was implanted percutaneously within the coronary sinus and the animals were followed 97±6 days (n=14) with monthly intracardiac echocardiograms. The MR grade (0-4) decreased from 3.1±0.2 at the time for implantation to 0.8±0.2 (p=0.0005) at the 3 month follow up. The vena contracta decreased from 6.5±0.4 mm to 2.0±0.7 mm after 3 months (p=0.0007). The mitral septo-lateral diameter was reduced after insertion of the device, from 38±1 mm before implantation to 35±1 mm after 3 months (P=0.0322). Angiography showed no signs of impairment of the coronary arteries. No thrombosis was observed.
Conclusion: These results indicate that experimentally induced ischaemic mitral regurgitation can be significantly reduced by means of a percutaneous catheter technique from the coronary sinus in surviving sheep.
Introduction
Functional mitral valve regurgitation (MR) is frequently present in the population of patients with ischaemic myocardial disease, thus up to 50% of patients having their first myocardial infarction (MI) develop MR1. Even mild ischaemic MR (grade 1 to 2) has been shown to be an independent predictor of cardiovascular mortality2, particularly in the presence of congestive heart failure2,3. A moderate to severe MR indicates a poor outcome even if the patient is asymptomatic, but the outcome is improved with early repair4. Surgical repair includes annulus reduction and enforcement by means of annuloplasty rings and bands, however, morbidity, mortality and late recurrent MR limits widespread use of such repairs5-9. Less invasive approaches than surgery could therefore be preferable. The coronary sinus (CS) and the great cardiac vein (GCV) run parallel to and adjacent to two thirds of the mitral valve. Attempts to reduce the mitral valve annulus from the CS in pacemaker induced cardiomyopathy in ovine experiments have been reported to be efficient10-12. Acute testing of a CS device in surgically induced ischaemic mitral regurgitation was presented by Liddicoat and Daimon13,14. Our aim in the presented study was to assess the efficacy of mitral annulus reduction from the CS in ischaemic MR in a chronic implantation study. We introduce a new method of creating ischaemic MR induced by selective injection of microspheres into the left coronary artery in sheep. A new device for treatment of MR by means of foreshortening of the CS and the GCV, thus reducing the septo-lateral diameter of the mitral valve has been developed by Edwards Lifesciences and was utilized in these experiments.
Methods
Ischaemic-induced mitral regurgitation
Thirty-seven sheep of a native domestic Swedish breed and a mean weight of 65±1 kg were used. All the animals received humane care in compliance with the “Principles of Laboratory Animal Care” by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” by the Institute of Laboratory Animal Resources, National Research Council, National Academy Press, 1996. Each animal was sedated with 5 mg Midazolam (Roche, Switzerland) and 500 mg Ketamine hydrochloride (Pfizer, Sweden) intramuscularly. The animal was secured on the operating table in dorsal recumbency. Anaesthesia was induced with thiopental (Abbot, USA) 5-8 mg/kg given in a peripheral vein and maintained with a mixture of O2 and Isoflurane (Abbott, Sweden). An infusion of Propofol (Braun, Germany), Fentanyl (Pharmalink, Sweden) and Ketamine was administered when applicable.
An endotracheal tube was inserted and mechanical ventilation initiated with tidal volumes set at 8-10 ml/kg body weight with a 100 ml compensation for dead space, at a rate of 12-15 cycles per minute. A gastric tube was inserted. Fluid support (Lactated Ringers Solution) was delivered intravenously. Capillary oxygen saturation was monitored and kept over 95% by means of ventilation volumes and inspiratory oxygen concentration. Non-invasive blood pressure was recorded every one minute and continuous ECG monitoring was done.
A femoral artery was cannulated and a 6 F sheath Medikit (Medikit Co. Ltd. Tokyo, Japan) was introduced. Through the sheath a diagnostic left coronary artery (LCA) angiogram was performed by means of a JL4 6F catheter (Boston Scientific Inc. Mapplegrove MN, USA). After assessment of the anatomy the largest side branch of the left coronary artery was catheterized with an OTW 2.5 x20 mm balloon Maverick (Boston Scientific) using a 0.014 inch PT-choice guide-wire (Boston Scientific). With the inflated balloon (6-atm) in the ostium of the side-branch the guidewire was removed and through the guidewire channel 1/2 to 1 ml microspheres Contour SE Microspheres 5-700 µm (Boston Scientific) which are provided in a suspension of alcohol (Boston Scientific, USA) was injected slowly within 1/2-1 minute. In some animals, when no large side branches were visible after previous treatments, the microspheres were injected in the main stem of the LCA, or in the ostium of the circumflex artery alternatively in the mid-portion of the left anterior descending artery. The microsphere injections were repeated with one month intervals until the resulting myocardial infarctions had caused left ventricular enlargement and mitral regurgitation. Prior to microsphere injection 2.5 ml of Lidocain 20 mg/mL was given intravenously as well as 1 ml of Tramadol. Intramuscular Tramadol, 0.5 mL, and a Paracetamol, 0.5 g suppository, were given after the procedure.
Anaesthesia was weaned and the animal was observed for 24 to 48 hours before returning to the farm.
Electrocardiogram
Before and after each treatment with microspheres and before device implant and at sacrifice a 12 channel electrocardiogram (ECG) was obtained (QRS Diagnostic, UK).
Echocardiography
When screening for pretreated animals eligible for device implantation, transthoracic echocardiography, TTE (Cypress, Accuson) was performed before and monthly after microsphere treatments until a significant MR was observed. The degree of MR was estimated on a scale from 0 to 4. A MR degree 2 or higher was considered significant and qualified the animal for device implantation4. The vena contracta was used as a quantitative measurement of the degree of MR. Five measurements of the mitral valve septo-lateral diameter were made each time, and the mean value of these was calculated. The left atrial diameter was measured, as well as the left ventricular diameter in systole and diastole.
A probe for intracardiac echocardiography, ICE, (AcuNav, 10F, Siemens, USA) was introduced via the external jugular vein into the right atrium at the time of device implantation, at one-month intervals and at sacrifice. A view from the right atrium just above the tricuspid valve was used for measuring the mitral valve septo-lateral distance.
The left ventricular volumes were calculated according to the formula: V=D3*7/(2.4+D), where V=left ventricular volume and D=left ventricular diameter. The ejection fraction (EF) was calculated according to the Teichholtz formula: EF=(EDV-ESV)/EDV*100, where EDV is the end-diastolic volume and ESV is the end-systolic volume.
The annuloplasty device
The mitral annuloplasty device, Viking (Edwards Lifesciences, Irvine, CA, USA) is made of Nitinol, a shape memory alloy of nickel and titanium. The device is intended for percutaneous, intravenous repair of mitral valve regurgitation by indirect remodelling of the mitral valve annulus from the CS and the GCV. The system is a catheter-deployed device consisting of a 10F diameter guide catheter, dilator, 8.8 F delivery catheter, and the therapy device. The system is compatible with a .035” guide wire. The annuloplasty therapy device itself consists of a coaxial over-the-wire delivery system with the self-expanding Nitinol annuloplasty device mounted in the distal segment (Figure 1).
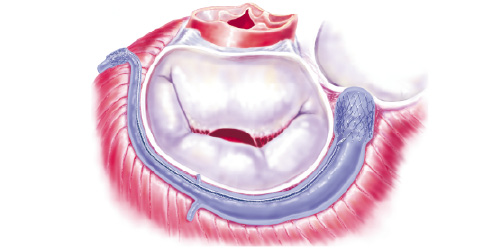
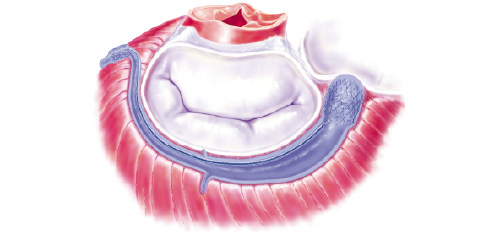
Figure 1. a) The Viking device is deployed in the coronary sinus and the great cardiac vein. The small anchor is placed in the anterior interventricular vein. The large anchor is placed at the orifice of the CS. The middle segment is still in its elongated phase. b) The middle segment contracts after 3-4 weeks. The septo-lateral diameter of the mitral valve is reduced, eliminating the mitral regurgitation.
The foreshortening middle segment of the device has spring-like properties due to the memory of the Nitinol alloy, and the stent-shaped end-segments serve as anchors. The memory of Nitinol is activated by body temperature. The middle segment is cut in a specific pattern leaving gaps in this segment. A biodegradable material fills the gaps and acts as a spacer thus keeping it in its elongated phase for 3-4 weeks. This period will allow secure incorporation of the anchors into the wall of the CS before contraction of the device begins. The device is provided in a number of different lengths and anchor diameters.
Deployment
In the anaesthetized animals a bolus of 7,500 IU of Heparin was given intravenously. A TTE was performed to confirm a significant MR. An electrocardiogram was taken. A 12 F introducer sheath Medikit (Medikit Co Ltd, Tokyo, Japan) was placed in the external jugular vein with Seldinger technique and an ICE was performed through this access. The guide catheter was introduced and placed in the orifice of the CS. Sheep have a left sided hemiazygos vein emptying into the CS close to the orifice. For this reason, the branching site was localized and the GCV catheterized with a JR4 6F catheter (Boston Scientific). A guidewire (0.035”, 260 cm, Glidewire Terumo, Japan) was advanced into the distal vessel. A measurement catheter (Royal Flush™, Cook Inc., Bloomington, USA) with radiopaque 1 cm markers was advanced over the wire to the mid-portion of the interventricular vein. The coronary venous system was opacified utilizing injections through the measurement catheter. The length of the vessel available for device implantation was determined by counting the radiopaque markers. The diameter size of the anchors were chosen using the guiding catheter as reference and oversized 1-2 mm. The guidewire was reinserted and the measurement catheter was withdrawn. The device delivery catheter was then passed over the wire, through the guiding catheter and into the coronary venous system. The device was placed with one anchor in the anterior interventricular vein, and the other at the orifice of the CS. After deployment of the device all catheters were retracted, the access routes were closed with manual compression, and the animal was allowed to wake up. After an observation period of one day the animal was returned to the breeder. ASA 75 mg (Trombyl, Pharmacia, Sweden) and Clopidogrel 75 mg (Plavix) was given daily throughout the study, starting on the morning before implant.
Follow-up
The animals were followed for 3 months and examined monthly with ICE, fluoroscopy and ECG under anaesthesia as described above. After 3-months, the animals were anaesthetized. ICE, fluoroscopy, LCA angiogram and ECG were performed before sacrifice. The sheep were sacrificed by means of an intravenous injection of potassium chloride and autopsied. An additional LCA-angiogram was performed on the explanted heart ex-vivo. The hearts were dissected and studied macroscopically with photo documentation and then preserved in formaldehyde for histopathological examination. Specimens were also taken from each lung for microscopy.
Analysis of data
All results are presented as means ± standard error of the mean (SEM), and n refers to the number of animals. The difference between all variables measured pre and post were analysed using the Wilcoxon Signed Rank test, with P values less than 0.05 being considered significant.
Results
Microsphere treatment
Of 37 microsphere treated animals 2 had not developed MR grade 2 on a scale of 4 after optimal microsphere treatment, even though the left ventricle had considerable enlargement, and were not included in the study; 21 animals died of the induced MI, either in connection with microsphere treatment or during the observation period before they were ready for device implantation. The survival rate was improved during the course of the study. The time to develop a MR > grade 2 averaged 130±16 days (n=14) and an average of 3.4±0.4 (n=14) treatments in one-month intervals were necessary.
Device implantation and clinical follow-up
Thus 14 animals were alive and had developed a significant MR after microsphere treatment and were eligible for device insertion. At implant the mean total procedure time was 61±6 min, whereof catheter intervention time was 27±3 min and the device-related implant time was 6±0 min (n=14). The mean follow-up time was 97±6 days (n=14). The only complication was a 0.014 inch guide wire perforation of the CS. A small amount of blood was seen in the pericardium at ultrasound, no treatment was necessary. All animals were up and standing immediately after the procedure. In one of the first animals, fluoroscopy revealed a separation in the bridge section near the small anchor at the one-month follow-up. Echocardiography demonstrated a MR grade 1 and the vena contracta was 2 mm. Thus the device was still effective and the animal was doing well. It was sacrificed in order to evaluate the cause of the separation. The cause was due to irregularities in the nitinol alloy, and corrective actions have later been taken to improve the device. The bridge was covered by firm fibrous tissue ingrowths, which could account for the effect despite separation.
Echocardiography
All echocardiographic measurements are given in Table I.
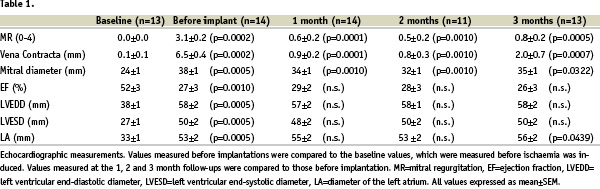
Microsphere treatment caused a significant mitral regurgitation, which was measured semi-quantitatively as an increased MR grade and quantitatively as an increased vena contracta. The mitral annulus diameter increased 58 % from 24 to 38 mm. The diameters of the left ventricle and atrium increased significantly and there was a significant reduction of the ejection fraction.
Treatment with the device caused a significant reduction of the degree of mitral regurgitation as well as the vena contracta. Both remained stable during the entire three month follow-up. The mitral valve diameter was also significantly reduced during the entire follow-up period. No mitral stenosis could be demonstrated. Left ventricular end-systolic and end-diastolic diameters as well as the ejection fraction did not change significantly. The diameter of the left atrium increased significantly after 3 months. Normal flow was seen in the CS with Doppler in all cases.
Angiography
Coronary angiograms in-vivo as well as one post mortem ex-vivo, were obtained in all animals. All angiograms showed normal left dominant coronary arteries, fully open with no signs of stenosis, neither in the proximity of the anchors, nor in the middle segment, next to the circumflex artery. The vein phase of the angiogram showed open coronary sinuses and great cardiac veins in all cases.
Macroscopic findings
All anchors were well healed into the wall of the CS, with no signs of thrombosis. The middle segment was firmly attached to the wall of the vessel and covered by a sheath of fibrous tissue and endothelium. In specimens from both lungs no pulmonary emboli were found.
Discussion
The surgical method of creating ischaemic MR through thoracotomy with coronary artery snares on marginal circumflex arteries has been previously described15,14. We chose to induce ischaemia and MI by microsphere injections in the side branches of the left anterior descending and the circumflex arteries. This was done repeatedly in one-month intervals. The method is time consuming, requiring in average 3.4 treatments per animal. The mortality rate with this model is similar to the previously described method14, the success rate for creating MR is higher however.
Though mortality was high during our initial experience with the microsphere treatment, we learned to use smaller amounts of microspheres and accept additional treatments. Limiting injections to either the diagonal artery system or the circumflex system during one treatment session, and avoiding injections in the proximal LAD, also resulted in better survival.
Mild or moderate MR is present in approximately 20% of the population16,17, the causes are degenerative, ischaemic or dilated cardiomyopathy. The patients with degenerative MR will remain surgical candidates in the near future. However, catheter based therapies are on the horizon. By means of clips or sutures the anterior leaflet may be fixed to the posterior, creating a double orifice and eliminating leaflet prolapse18,19. Most surgical repairs of the MV include an annuloplasty ring, even when tailoring of the leaflets has been performed. For surgical repair of pure functional ischaemic MR an annuloplasty ring alone is the preferred treatment. The rationale for developing annuloplasty devices from the CS is based on the favourable anatomical relationship between 2/3 of the mitral valve annulus and the CS and its major first order tributary, the GCV. The cardiac veins are delicate structures; the device was therefore designed to have its action only after the anchors have healed firmly into the venous walls. A multiple number of device sizes are necessary to get a perfect fit. The foreshortening distance necessary was calculated from the effect achieved on the MV annulus when surgical annuloplasty rings were inserted. The plane of the CS and the GCV is almost parallel and close to that of the MV, located in the atrioventricular groove. Anatomical variation of the course and size of the utilized veins is relatively frequent. However, 3-D CT cardiac reconstructions in the human safety and efficacy study that has just begun reveal that the majority of patients have cardiac veins suitable for the device. The GCV crosses over the circumflex artery or one of its branches regularly. There has been concern that cardiac vein devices would compromise these arteries. In dogs, Mainu et al observed such artery compression when the anchor was placed directly over the artery11. We place the anchors with good margins to the arteries, and the foreshortening bridge-part crosses frequently the circumflex artery. We did not in this study, in a pacemaker study12 nor in a safety study with surviving animals for 6 months observe any compression of the arteries. One explanation could be that the foreshortening of the device is not acute; the effect comes gradually after 3 weeks and is slow in action, in this way allowing adaptation to the stress inflicted.
Since the pathophysiology and the anatomical conditions of mitral valve regurgitation are so different from case to case, it is most unlikely that any of the percutaneous MV devices will be the sole solution for all patients. More likely they will be complementary to each other, e.g. one percutaneously attached suture or a clip on the leaflet18 in conjunction with an annuloplasty device in the venous system.
In conclusion, this study shows that ischaemic mitral valve regurgitation, induced by selective microsphere injections into left coronary artery branches, can be successfully treated by means of the presented device placed in the cardiac vein system of sheep, and that the effect on the valve is consistent over time.
Acknowledgments
We thank Åsa Klitthammar R.N.F.A for all her help with these experiments.
Grant sponsor: Edwards Lifesciences, Irvine, Ca, U.S.A.

