Abstract
Aims: To develop a percutaneous, coronary sinus-based mitral annuloplasty system that includes both diagnostic and implant capabilities and that enables late adjustment of annuloplasty effect.
Methods and results: A multi-lumen catheter and an array of annuloplasty devices for coordinated insertion comprise an annuloplasty system that has been validated in extensive pre-clinical diagnostic and implant testing. Initial human diagnostic studies with this percutaneous transvenous mitral annuloplasty (PTMA) system demonstrate feasibility of annuloplasty via the coronary sinus and confirm the ability to alter mitral valve geometry and function via instrumentation of the coronary sinus.
Conclusion: PTMA is feasible and promises to offer a new clinical alternative to patients with heart failure and functional mitral regurgitation. PTMA may also be employed to supplement percutaneous repair techniques that address the mitral leaflets.
Introduction
During the last two decades, percutaneous, endovascular therapies have revolutionized the treatment of coronary artery disease. However, the development and dissemination of percutaneous approaches to heart valve disease have been less rapid. While rheumatic mitral stenosis and congenital aortic stenosis are effectively managed by balloon valvuloplasty in many instances1,2 and pulmonary regurgitation can be treated by percutaneous pulmonary valve insertion3, progress with more common valvular lesions-mitral regurgitation (MR) and acquired aortic stenosis-has required development of novel technologies.
MR is the most common valve lesion encountered in clinical practice. Valve dysfunction in patients with MR may be caused by a variety of disorders, including degenerative, rheumatic, endocarditic, congenital, ischaemic, or functional processes. In patients with functional MR of ischaemic or cardiomyopathic origin, regurgitation is caused by changes in left ventricular and mitral annular geometry and function that prevent normal leaflet coaptation. Patients with congestive heart failure (CHF) are particularly prone to functional MR. It is estimated that 15% of CHF patients have clinically important MR, meaning that worldwide there are approximately 3,000,000 CHF patients with MR.
In patients with functional MR, surgical repair is achieved by annuloplasty. However, because of morbidity and mortality associated with operation, few of these CHF patients come to surgery; therefore, they represent a logical target for development of percutaneous therapies to address mitral valve dysfunction. Recognizing that the coronary sinus courses in the atrioventricular groove in close proximity to the mitral annulus, we hypothesized that it would be possible to develop a percutaneous, coronary sinus-based procedure that would achieve effects similar to those of a surgical annuloplasty. Such a procedure would offer the possibility of safe, minimally invasive mitral valve repair to patients with functional MR and CHF.
Percutaneous transvenous mitral annuloplasty device
The Percutaneous Transvenous Mitral Annuloplasty (PTMA) system (Viacor, Inc, MA) includes a custom 7 Fr implant catheter that has 3 lumens (Figure 1).

Figure 1. The percutaneous transvenous mitral annuloplasty (PTMA) implant device. The proximal hub provides for sealed, re-accessible access to the internal PTMA implant rods. Implant rods are selected based upon the diagnostic PTMA procedure.
This catheter is introduced into the coronary sinus via the subclavian or jugular vein using standard access techniques. The tip of the 7 Fr catheter is positioned in the anterior interventricular vein under fluoroscopic guidance.
Annuloplasty devices are introduced into the coronary sinus through the lumens of the custom catheter using fluoroscopy and echocardiography. Each annuloplasty device is fabricated from a single piece of drawn nitinol wire to a particular length and stiffness profile (Figure 2).
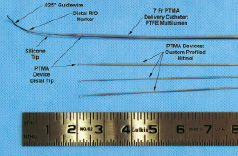
Figure 2. Distal configuration of the diagnostic PTMA system. The multi-lumen diagnostic catheter is delivered to the coronary sinus-great cardiac vein continuity using standard over-the-wire technique. Up to three diagnostic PTMA rods are selectively placed within the catheter to temporarily reduced the anterior-posterior annular dimension and allow for selection of the appropriate implant device sizes.
By varying the number of devices and stiffness of each device placed in the custom catheter, the total stiffness can be magnified six fold, increasing the force delivered to the posterior mitral annulus. When positioned in the coronary sinus, the annuloplasty devices effect a graded conformational change in the mitral annulus that reduces AP diameter and increases leaflet coaptation. Devices are added or interchanged systematically in the 7 Fr custom delivery catheter to optimize reduction in anterior-posterior (AP) mitral annular dimension and MR degree on echocardiography.
The procedure involves both diagnostic and treatment stages. Using a diagnostic 7 Fr multi-lumen delivery catheter the impact of PTMA is assessed with echocardiography (Figure 2). The optimum combination of annuloplasty devices is recorded, and the diagnostic multi-lumen catheter is exchanged for an implant catheter using standard, over-the-wire techniques (Figure 1). After the PTMA implant catheter is in place, the pre-selected annuloplasty devices are placed in its lumens, and the impact on MR confirmed by echocardiography. The hub of the catheter is sealed and placed in a small subcutaneous pocket at the site of percutaneous access. Alternatively, if the diagnostic procedure demonstrates insufficient response to PTMA, the diagnostic catheter is removed and no implant is delivered.
Late adjustment of treatment effect is achieved by opening the subcutaneous pocket and accessing the 7 Fr multi-lumen delivery catheter. Under echocardiographic guidance, PTMA devices may be added or removed, altering mitral annular geometry to optimize reduction in MR.
Preclinical studies
We have performed acute and chronic diagnostic and implant studies in more than 300 sheep. These include investigations in normal sheep, sheep with ischaemic MR, and sheep with CHF and MR induced by rapid pacing.
In normal sheep, we have demonstrated reductions in mitral annular diameter of 5 to 12 mm, mimicking the changes observed with annuloplasty in humans. In sheep with acute ischaemic MR, PTMA dramatically changed mitral annular geometry with a resultant decrease in MR.4 Employing 2 and 3 dimensional echocardiography to study sheep with chronic ischaemic MR, we found that PTMA reduced MR jet area, mitral annular AP dimension both in systole and diastole and mitral valve tenting area.5 We did not observe new ischaemia in the distribution of the circumflex coronary artery. Chronic studies of the permanent implant have confirmed maintenance of the annuloplasty effect and the ability to re-access the multi-lumen catheter and adjust the annuloplasty up to 6 months after implantation. Pathologic studies demonstrate preserved patency of the coronary sinus and great cardiac vein with no damage to the mitral valve or circumflex coronary artery with chronic implantation (Figure 3).
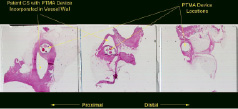
Figure 3. Histopathology of a 50 day implant. Three cross-sectional views of the coronary sinus from proximal to distal, with the catheter and the three PTMA devices in place.
Human experience
As of this writing, diagnostic PTMA (without implantation) has been performed in 10 patients undergoing open heart surgery for treatment of functional MR. Under this protocol, investigators had approximately 1 hour to perform diagnostic PTMA. Initial experience with a single lumen prototype device in 5 patients confirmed the feasibility of alteration of mitral annular geometry. In the next 5 patients, the multi-lumen device was employed; successful delivery occurred in 4 patients, with reduction of MR by 2 grades in 2 patients and no treatment effect in 1. Of note, in the patient with no treatment effect, surgical annuloplasty also failed to reduce MR, and the patient underwent mitral valve replacement. In the fifth patient, there was reduction in MR grade by PTMA, but alterations in systemic blood pressure rendered assessment of treatment effect problematic.
Discussion
These extensive preclinical studies coupled with early clinical experience document development of a percutaneous, coronary sinus-based mitral annuloplasty system that includes both diagnostic and implant capabilities and that enables late adjustment of annuloplasty effect. Successful human application of such a technology has important clinical implications.
Functional MR is a major and growing clinical problem. Recent reports document that 7,800,000 Americans suffer myocardial infarction annually;6 19% of these can be expected to develop significant MR.7,8 Although mitral valve dysfunction increases symptoms of heart failure and reduces survival in these patients, the MR is rarely addressed directly. Surgical annuloplasty is the only current option for treatment, but this operation is associated with high morbidity and mortality, limiting its application.9-11 However, patients who survive surgery frequently derive clinical benefit from the surgical annuloplasty.
Several potential mechanisms, including subvalvular papillary muscle displacement which causes leaflet tenting, contribute to malcoaptation of the mitral leaflets in chronic functional MR.12-14 In spite of this mechanistic complexity, annuloplasty alone produces satisfactory reduction of ischaemic MR in 70% to 80% of cases.15,16
There is currently great interest in use of the coronary sinus to facilitate minimally invasive mitral annuloplasty. Percutaneous placement of mitral annuloplasty devices has been reported to reduce MR in models of global left ventricular dysfunction17 and acute and chronic ischaemia.4,5 The ideal annuloplasty device includes both diagnostic capability and the possibility of late adjustment. It is clear that not all patients with functional MR can be managed successfully by annuloplasty. A diagnostic procedure before permanent implantation will help to identify those patients who respond favorably to PTMA. Surgical series demonstrate that after an initial favorable response to annuloplasty, up to 30% of patients develop recurrent MR16. With PTMA, late adjustment of annuloplasty effect may enable continued successful treatment of MR in such patients.
Remaining questions
Patient selection is currently unclear. Pre-procedure anatomic imaging, likely by CT scan, is necessary to define the anatomic relationships of the mitral valve and coronary sinus that are most favorable for PTMA. Similarly, 2 and 3 dimensional echocardiography may help to identify mitral valves most likely to be treated successfully by PTMA. Once patient selection is clarified, clinical studies will be necessary to document the long-term safety, efficacy, and clinical impact of PTMA. While data from surgical series suggest a benefit from annuloplasty in functional MR, the magnitude of this effect and the survival impact remain controversial. Because the overwhelming majority of patients with CHF and functional MR are treated medically rather than surgically, assessment of PTMA will likely require comparisons between patients receiving best medical therapy and those receiving both PTMA and best medical therapy. Design of such trials is currently underway.
Acknowledgments
Dr. Liddicoat is a former Director of Viacor, Inc. and has an equity interest.
Dr. Gillinov is a consultant to Viacor, Inc. and has an equity interest. Dr. Gillinov is also a consultant to Edwards, Lifesciences, LLC.

