This article reviews aetiology, natural history, and current treatment options in hypertrophic cardiomyopathy with a special focus on percutaneous septal ablation, which has evolved as an alternative to myectomy in patients with outflow obstruction and symptoms refractory to medical treatment. Literature data and the own series of 382 interventions (in-hospital mortality and pacemaker implantation rates: 1.2% and 7%, respectively) are discussed. Overall satisfactory clinical results were seen in about 90% of the patients treated with septal ablation.
Introduction
Hypertrophic cardiomyopathy (HCM) is a cardiac condition morphologically characterized by unexplained myocardial hypertrophy. The prevalence of the disease is considered to be 0,1-0,5%, obstruction to left ventricular outflow being present in about 20-30% of affected patients2,28,48,55. During the past decade, treatment options for HCM have changed dramatically, both with respect to risk management, and improvement of symptoms.
Aetiology, pathogenesis, and pathophysiology of HCM
In >50% of patients, HCM has a familiar background. Inheritance is monogenetic, autosomal-dominant, with a highly variable penetrance. Mutations have been found in >10 genes coding for sarcomeric proteins; the condition therefore has been characterized as a “sarcomeric disease”2,9,28,48,50,55, with impaired generation of contractile force on the intra-cellular level. Recently, however, this concept was challenged by the finding of mutations in non-sarcomeric proteins being also associated with a HCM phenotype. Histologically, the prominent findings in HCM are myocardial disarray, hypertrophy, and increased fibrosis48,55. The coronary arteries are also involved showing wall thickening and frequently myocardial bridging. Additionally, mitral valve leaflets may be elongated55. One concept of HCM pathogenesis is that of a “defect healing” with the molecular dysfunction as the primary abnormality, and the hypertrophic process as the compensation. Left ventricular systolic function is indeed normal in the vast majority of patients. Myocardial fibrosis and hypertrophy, however, lead to increased myocardial stiffness and impairment of diastolic left ventricular function early in the disease process48,55.
Independent from functional limitations, supraventricular and ventricular arrhythmias may occur. Again, fibrosis and disarray are considered as the arrhythmogenic substrate; myocardial ischemia due to hypertrophy and thickened vessel walls may be a trigger2,28,48,55. Sudden cardiac death due to ventricular tachyarrhythmias is a feared complication of the disease, and sometimes its first manifestation. Among young (<35 years) athletes dying suddenly, HCM is considered to be responsible in about 30%2,28. The dissociation between morphology, functional status, and arrhythmogenic risk is a major problem of HCM management2,28,48,55.
An important differential diagnosis is that between the non-obstructive (hypertrophic nonobstructive cardiomyopathy: HNCM) and the obstructive (HOCM) variant of the disease. Dependent on the distribution of hypertrophy within the left ventricle, the septal curvature, the configuration of the mitral valve, and left ventricular loading conditions, about 20-30% of HCM patients develop a dynamic obstruction between a “high-pressure” and a “low-pressure”compartment of the left ventricle55. Typically this obstruction occurs between the subaortic septum and parts of the mitral valve (“SAM”-phenomenon: systolic anterior movement), and is associated with mitral regurgitation55. In a minority of cases it may be located in the midcavity region, or in the apex. Figure 1 shows typical echocardiographic examples of non-obstructive and obstructive HCM.
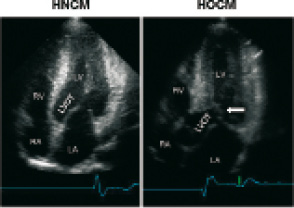
Figure 1. Echocardiographic differentiation between non-obstructive (HNCM, left) and obstructive (HOCM) hypertrophic cardiomyopathy (arrow: SAM phenomenon). LA: left atrium, RA: right atrium, LV: left ventricle, RV: right ventricle; LVOT: left ventricular outflow tract.
The haemodynamic significance of obstruction seems to depend on the size of the LV compartment that is working against increased afterload (subaortic>midcavity obstruction); apical gradients are considered to be less significant55. The pathphysiology of obstruction was heavily debated upon earlier; the current concept combines a “trigger” (= vigorous ejection during early systole) with a “substrate” (= anatomy of the subaortic septum, the outflow tract, and the mitral valve55). Not surprising, a substantial degree of variability has been described regarding gradient severity, and provocation (by physical exercise, preload reduction, or post-extrasystolic augmentation) is essential to distinguish between HNCM and HOCM both during echocardiographic and invasive haemodynamic studies2,28,48,55.
Symptoms, clinical workup, and natural history in HCM
Typical symptoms in HCM patients are dyspnoea, angina, or dizziness on exertion. Palpitations or syncope occurring both with and without exercise are reported by 20-30%. Recurrent syncope and a family history of sudden cardiac death (at <45 years) are considered risk factors2,8,28,48,55. Overall, a very variable degree of limitation is characteristic. On the other hand, a severe phenotype does not necessarily preclude normal exercise capacity, or even athletic performance.
Physical examination is usually normal in patients with HNCM; the characteristic finding in HOCM is the variable systolic murmur which accentuates with preload reduction. The diagnosis can usually be made non-invasively by imaging techniques (echocardiography, cardiac MR, multislice CT); the examiner should be prepared to see highly variable hypertrophy patterns. A wall thickness of >30 mm has to be actively looked for since this seems to be another risk factor for sudden cardiac death2,8,28. Provocative maneuvers are essential to differentiate HNCM and HOCM. Typical ECG changes are “giant negative T waves” in HNCM and “pseudo-infarction Q waves” in HOCM, however, all types of ECG changes may be present. Holter monitoring should be performed for risk stratification (non-sustained VT’s). Stress testing is useful to measure the degree of functional limitation, and to check the blood pressure response to exercise which is considered another risk factor for sudden cardiac death8.
Invasive studies are needed to exclude coexistent coronary artery disease, to visualize the anatomy of the septal perforator arteries if septal ablation is considered, and to perform endomyocardial biopsy if a myocardial storage disease is suspected. The level of suspicion for such a storage disease should be high in presence of a low-voltage ECG. A prevalence of storage diseases of up to 10% has been reported in “HCM” series2,28. Diastolic LV performance and the outflow gradients can also be assessed invasively; in our patient cohort, matching with the respective echocardiographic findings was reliable. The role of invasive electrophysiology studies for risk stratification is uncertain2,28.
Natural history in HCM is again highly variable2,28,48,55. In most cases the diagnosis is made during adolescence and early adulthood, and symptoms are slowly progressive. Disease manifestation in childhood is considered prognostically ominous. Late manifestation, however, is typical in carriers of the myosin-binding protein C mutation2,9,28. Prognosis is determined by arrhythmic events in younger patients, and typically independent from symptoms in this group, and by cardiac failure and stroke in elderly patients. In non-selected cohorts, the annual mortality rate is reported to be around 1%/year, in high-risk group this figure rises up to 5-6%2,28,48.
Therapeutic options for obstructive HCM
General considerations and risk stratification
Whether or not obstruction or symptoms are present, HCM patients should not engage in competitive sports2,28. A limitation with respect to moderate physical activities in asymptomatic patients, however, does not seem to be justified. Outflow obstruction may exacerbate with alcohol intake, and includes an increased risk for infective endocarditis, prophylaxis is thus recommended. HCM patients in atrial fibrillation are especially prone to thromboembolic stroke, oral anticoagulation is mandatory in these cases2,28.
All HCM patients should be risk-stratified, since the implantation of an ICD reliably reduces arrhythmogenic cardiac events. Risk stratification is based on a “malignant” family history (- of sudden cardiac death at <45 years), recurrent syncope, non-sustained ventricular tachycardia on Holter monitoring, inadequate blood pressure rise with exercise, and excessive LVH (>30 mm). In individuals with two or more of these risk markers, ICD implantation should be considered2,8,28.
Medical therapy
Medical therapy with negative inotropic drugs (beta blockers, calcium antagonists of the verapamil type, disopyramide) is the first line of treatment in order to reduce symptoms and improve quality of life2,28,45. The influence on obstruction is moderate, mostly due to reduction of the “trigger” (- forceful LV contraction during early systole). Additional anti-fibrillatory effects may be present for beta blockers, while verapamil is supposed to have a positive effect on diastolic LV function. The effect of disopyramide on obstruction seems to exceed that of the two other drugs45. The anti-arrhythmic properties of drug treatment are welcome in many patients, on the other hand, latent conduction abnormalities may exacerbate in individual cases. Furthermore, about 5-10% of pts. may have a paradoxical haemodynamic response to verapamil. The initiation of treatment with verapamil and disopyramide therefore should be monitored closely. Overall, in many pts. the effect of drug treatment vanishes over the years, and none of these strategies is really “evidence-based”. Drugs that lead to a marked pre- or afterload reduction, or those with positive inotropic effects are contra-indicated in HOCM since they may produce drastic exacerbation of obstruction and haemodynamic collapse.
Surgical therapy
Surgical myotomy/myectomy, developed in the late 50ies and 60ies, traditionally has been the treatment of choice for patients with drug-refractory symptoms and significant (>50 mm Hg at rest) outflow tract obstruction2,6,7,15,21,29-33,35-37,39,40,48,49,51,56. The procedure primarily aims at the “substrate” of obstruction, i e. the protruding septal myocardium, leaves a left bundle branch block on surface ECG in >50% of the patients treated34,49 and usually a clearly visible septal trough on imaging studies. The depth and extent of septal resection can be tailored to the individual anatomy, thus also adressing mid-cavity obstruction or papillary muscle abnormalities if present7,29,31,51. Furthermore, valvular correction/replacement or coronary bypass grafting can be combined with the reduction of septal myocardium if necessary.
Reports on >2000 patients undergoing (isolated) myectomy consistently demonstrated clinical and haemodynamic success rates of >90% together with operative mortality rates that finally were reduced to <1-2% in experienced centers. Peri-operative monitoring by transesophageal echocardiography has become a routine procedure2,28-33,35-37. The rate of pacemaker dependency is reported to be <5%. A favourable effect on the hypertrophic process and a positive prognostic influence28,30,33,56 are suspected from long-term observations of post-myectomy patients; however, a randomized study against medical treatment does not exist. Favourable results of myectomy were also reported in specific subsets including pediatric patients30 as well as cases with atypical or midcavity obstruction2,7,28. Taken all this together, myectomy has set the standard of safety and efficacy of treatment for symptomatic obstructive HCM; and all alternatives should be measured against this standard2,22,28.
AV sequential stimulation
Dual-chamber pacemaker implantation was introduced as a less invasive alternative to myectomy17,27. Pacing from the RV apex may be understood as a combination of “trigger” reduction with a certain degree of “substrate” modification, i. e. a global negative inotropic effect and some outflow tract opening due to delayed activation of the basal septum. It also induces a left bundle branch block pattern; a gradient reduction of 50-90% has been reported17. Enthusiasm for this approach, however, was tempered since a considerable placebo effect became obvious in several randomized trials. At present, we consider AV sequential pacing a “niche indication” for
1. patients with left bundle branch block (- and thus a very high risk for complete AV block during septal ablation [see below]),
2. patients who need an ICD for risk reduction anyway, and
3. selected patients with isolated midcavity obstruction.
Septal ablation
From 1995 onward, the therapeutic options for HOCM have dramatically changed by the introduction of percutaneous septal ablation3-5,10-14,16,18-20,23-26,38,41-44,46,47. This procedure produces a circumscript septal infarction, leaves a septal lesion that often is undistiguishable from a myectomy trough, thus also aims at the “substrate” of obstruction, and reproduces the haemodynamic effect of a surgical myectomy10,11,16. About 60% of the patients treated show a right bundle branch block on surface ECG34,49, and have transient complete heart block during the procedure. The pacemaker rate varies between 5% and >20% in some centers; in patients with pre-existing left bundle branch block (see above) it has been reported to be as high as >60%12,34,49. Hospital mortality figures are comparable now to those in the best myectomy series (1-2%, see table 1).
Several acronyms are in use (in alphabetical order: alcohol septal ablation (ASA), non-surgical myocardial reduction (NSMR), percutaneous transluminal septal myocardial ablation (PTSMA), or transcoronary ablation of septal hypertrophy (TASH), reflecting different procedural strategies (mainly with or without intra-procedural echocardiography;2,10-14,18-20,24,25,28). Overall, during the past decade septal ablation has gained wide acceptance as the non-surgical alternative of choice for patients with hypertrophic cardiomyopathy, significant outflow obstruction, and symptoms refractory to medical treatment. About 3000 cases have been reported in the literature. However, these figures have lead to concerns suggesting that the threshold to perform a septal ablation procedure has been lowered inadequately2,28.
Septal ablation procedure
A detailed description of the technique has been repeatedly published by our and other groups, differing in several technical aspects4,10-14,18-20,24-26,53,54. There is consensus that a temporary pacemaker lead is to be inserted in all patients. The LVOT gradient is constantly monitored by simultaneous pressure recordings from the left ventricular apex and the ascending aorta. An over-the-wire balloon catheter is introduced into the target septal branch presumed to be responsible for the blood supply to the septal area involved in obstruction. The balloon is inflated, and the effect on obstruction measured (Figure 2).
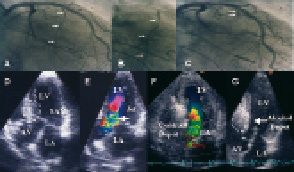
Figure 2. Upper panel: Left coronary angiography with the target septal branch (arrow) in RAO projection (A, arrows). Occlusion of the septal branch by balloon inflation and injection of dye through the central balloon lumen of the inflated balloon to visualize the supply area of the septal branch (B; arrow) and to exclude leakage into the LAD. Final visualization of the occluded septal branch (arrow) after alcohol injection (C). Lower panel: Echocardiographic study during PTSMA. Baseline view, here with an angulated apical 4-chamber view (D), where the point of SAM-septal contact (dotted line: target area) and the obstructive jet (E) can be easily identified (arrow). Injection of echo-contrast agent (F) with satisfactory opacification of the target area (arrow). Visualisation after the alcohol injection (G) LA: left atrium, LV: left ventricle, RA: right atrium, RV: right ventricle
In contrast to other techniques who strongly rely on the effect of balloon-induced ischemia on gradient severity4,18-20,24,25, in our practice as well as in most other centres the correct vessel selection is assured by way of injecting 1-2 ml of a non-toxic echocardiographic contrast agent (e. g. Levovist”, Schering, Berlin, Germany; 350 mg/ml) through the central lumen of the balloon catheter under simultaneous transthoracic echocardiographic monitoring. This approach exactly shows the septal area that will be attacked, i. e. the future area of necrosis (Figure 2). Opacification of any other cardiac structure has to be securely excluded11,26. Currently, in about 10% the procedure has to be stopped based on echocardiographic findings (- usually contrast in areas distant from the septal target region), and a target vessel change is necessary for the same reason in 10-15%11.
Only if the target region is correctly marked by the echo contrast agent, 1-3 ml of 96% alcohol (i. e. 1 ml per 1 cm of septal thickness) are slowly injected through the central lumen of the balloon catheter under analgesic medication (5-10 mg of morphine). The ethanol dose was substantially reduced by all groups over the years. Retrospective dose finding studies from the own and other series have shown that the main effect of higher ethanol doses is pronounced septal thinning, with a minor effect on gradient reduction12,42,53,54. Ten minutes after the last alcohol injection the balloon is deflated and removed, ensuring that no alcohol backwash occurs into the left anterior descending artery. A final angiogram excludes LAD damage and verifies septal branch occlusion, and a final haemodynamic measurement is performed.
The duration of post-interventional monitoring is controversially discussed. Since an artificial myocardial necrosis has been created, we suggest a monitoring duration of at least 48 hours (coronary or intensive care unit), with enzyme and ECG controls every 4 hours. Transvenous and transcutaneous pacing equipment should be readily available. After a risk evaluation based on the peri-interventional evolution, the duration of further monitoring can the be decided on12.
Patient selection for septal ablation
Criteria for patient selection follow largely those established for septal myectomy. Septal ablation may be considered as an alternative to septal myectomy in2,28,48,55:
1. Patients with symptoms limiting daily activities (Functional class >II, exercise-induced syncope) despite adequate medical treatment, or if medical treatment is not tolerated. Patients in functional class II are accepted in our practice on an individual basis if objective measurement of exercise capacity point to a more severe limitation than subjectively perceived.
2. Patients with a substantial degree of outflow obstruction (pressure drop >30mm Hg at rest or >100 mm Hg with provocation by a Valsalva maneuver, bicycle stress, or post-extrasystolic augmentation)
3. Patients with a suitable left ventricular and coronary morphology, i. e. those with a “classical”, subaortic obstruction produced by the protruding septum and the “SAM” of the mitral valve, and one or more septal perforator arteries that go to this septal area.
Patients with co-existing, significant coronary artery disease in one vessel only may be treated percutaneously first; ablation should be delayed until documentation of a good long-term result of PCI. In cases with multiple (>1) vessel disease, we prefer a surgical approach. In atypical obstruction or midcavity obstruction, the decision must be individualized; ablation is possible43 but results are less favourable in this subgroup as compared to subaortic obstruction. Patients without obstruction or without symptoms are not candidates for septal ablation2,28. At present, with respect to the very limited long-term experience with septal ablation (see below), and the favourable results of myectomy also in this age group30, we do not think that ablation should be performed in the pediatric population with obstructive HCM1,2,28.
A routine pre-interventional workup includes exercise testing and risk assessment with respect to the need for implantation of an automatic defibrillator; in patients who receive an ICD, pacing is tried first to reduce outflow obstruction (see above). A diagnostic left heart catheterization and coronary angiogram is performed to exclude significant coronary heart disease or another pathology that would require cardiac surgery.
Current results of septal ablation
1. SHORT TERM RESULTS
Across all reported series including the learning curve of the individual groups of investigators, septal ablation has a peri-procedural mortality of 1-4%, at present 1-2%. This holds true both for several single-centre series and for the one existing multicentre registry14,25,46. The injected ethanol doses gradually decreased over the years (from >5 to 1-3 ml), leading to smaller infarctions and less AV conduction problems42,53,54. However, the rate of pacemaker implantation still varies considerably (between <5 up to 20%). Following a local remodeling process, the morphologic and haemodynamic treatment result should be judged no earlier than after 3-6 months. At that time point, gradients usually are reduced by 80-90%, associated with an increase in exercise capacity by 20% and an improvement of diastolic LV function markers10,52. Table 2 gives an overview on short-time evolution (<12 months) of symptoms and obstruction in a selection of studies published over the years.
Our own series now comprises 382 patients (selected from 1005 patients evaluated and treated in our HCM clinic) in whom ablation was attempted between 1996 and 2005. Out of these, 342 patients (90%) received an average dose of 2.7±1.2 ml ethanol. Doses of >5 ml or injections into >1 septal perforator were no longer used from 1997 onward. In 40 patients the intervention was aborted without ethanol injection, mostly for safety reasons / due to contrast echocardiographic findings. CK peak was 537(259 U/l with an MB fraction of 64±35 U/l (normal values: <80 and <15 U/l, resp.). Transient AV conduction problems occurred in 181 pts. (53%); permanent AV sequential pacing was required in 25 pts (7%). Peri-interventional mortality was 1.2% (4 deaths). A complete 3 months follow-up at present is available for 312 patients (91%). Self-reported exercise capacity improved in 279 pts. (89%); 164 pts. (53%) reported to be free of symptoms. Average NYHA functional class improved from 2.9±0.4 to 1.5±0.6 along with a significant increase in objective exercise capacity (from 94±51 to 115±43 watts, p<0.01). The gradients were reduced from 60±32 to 13±18 mm Hg at rest, and from 121±44 to 39±35 mm Hg with provocation (p<0.0001); 121 pts. (39%) were free from outflow obstruction both at rest and with provocation. Septal thickness (from 20±4 to 17±4 mm; p<0.01) and left atrial diameter (from 49±7 to 46±7 mm; p<0.01) were reduced, while there was a slight increase in enddiastolic LV diameter. LV dilatation exceeding the individual normal value, or a global deterioration of systolic LV function, was not observed.
The limited number of non-randomized comparisons between septal ablation and (isolated) myectomy in general showed comparable clinical and haemodynamic results, with a slightly more pronounced improvement with respect to obstruction and exercise capacity following surgery, and different surface ECG patterns (ablation: RBBB; myectomy: LBBB) after intervention15,32,34-36,49,51. Whether these differences are clinically important, is unknown. A difference that may be important is fact that relief from obstruction is usually rapid after myectomy, whereas LV “unloading” after ablation may need several months.
2. LONG-TERM RESULTS
To our best knowledge, the only publication on long-term effects of septal ablation comes from our own group13 (Figure 3).
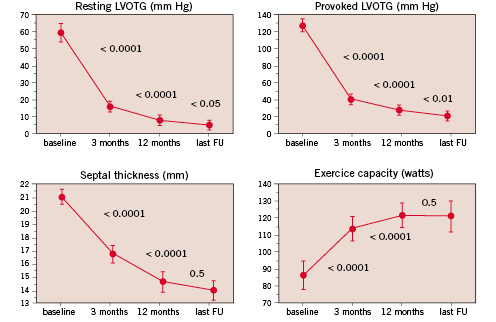
Figure 3. Evolution of septal thickness, outflow gradient at rest and with provocation, and exercise capacity during long-term follow-up after septal ablation.
The main findings of this study were for one that reduction of septal thickness and outflow gradient seems to continue over a 12-months period, presumably due to ongoing fibrosis and shrinking of the ethanol-induced lesion. The highest net gain in outflow tract diameter due to septal reduction occurred between 3- and 12-months of follow-up. Secondly, progressive LV dilatation was not observed, thus the remodeling process seems to remain limited to the region of intervention. Not only septal hypertrophy decreased as a consequence of the therapeutic infarction, but also left ventricular posterior wall thickness due to relief of the pressure overload, which in turn indicates that the hypertrophic process in HOCM may not be completely independent of LV afterload. A recent short-term study using magnetic resonance imaging confirmed this finding52.
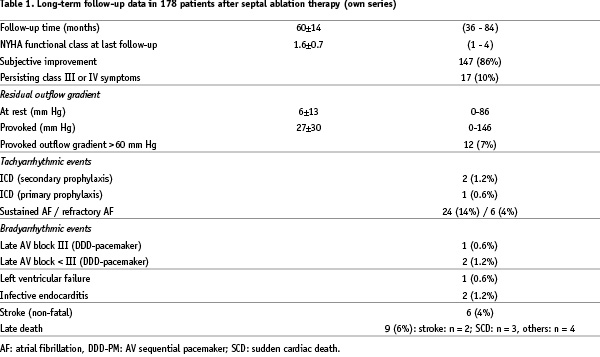
Since that publication, we repeatedly updated our “long-term cohort” of those 178 patients that were treated 1996-1998. Haemodynamic and clinical effects were maintained over a follow-up period of actually 60±14 months. Overall survival was 94%; event-free survival in NYHA class II or lower 73% (Figure 4).
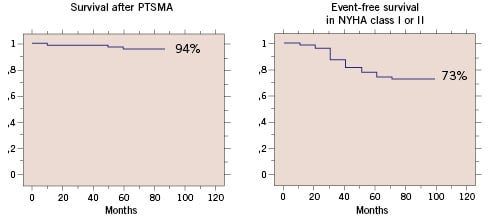
Figure 4. Survival (left) and event-free survival in NYHA class I or II (right) after septal ablation.
The most frequent clinical problem was atrial fibrillation. Overall survival and event free survival in our series were comparable to the reported post-surgical results33,37,39,40,56. Since both treatment options predominantly target the outflow gradient, it can be speculated that effective gradient reduction may be the reason for this positive outcome.
Conclusion
In experienced institutions, about 90% of the patients with severely symptomatic HOCM of the subaortic type can be treated effectively with septal ablation, making this procedure an attractive alternative to the “gold standard” of surgical myectomy. The procedure
a. requires a careful patient selection,
b. should be part of a comprehensive program for HCM patients which offers all other options (medical treatment, myectomy, pacemaker and ICD implantation)
c. results in a significant and long-standing clinical and haemodynamic benefit,
d. appears to have an acceptable safety profile.
Further long-term observations in large patient groups are necessary to define the role of septal ablation in comparison to surgical myectomy.

