Abstract
Percutaneous pulmonary valve implantation was successful in 83 patients. There was a significant clinical and functional improvement as assessed by subjective and objective means. Early procedural and device-related complications were encountered contributed to by the learning curve and remedied by increasing clinical experience and improvement of device-design. In well-selected patients, percutaneous pulmonary valve implantation is a safe and effective alternative technique for treating right ventricular outflow tract dysfunction.
Introduction
We present here an update on the clinical experience of Percutaneous Pulmonary Valve Implantation (PPVI)1. Since its inception in September2, 2000, 83 consecutive patients had pulmonary valve implantation by transcatheter technique resulting in up to 5 year follow-up data from the first implant. All patients had surgery on their Right Ventricular Outflow Tract (RVOT) to repair congenital heart disease, as a re-operation during late follow-up or during surgical intervention on the left ventricular outflow tract. The presence of a right ventricle to pulmonary artery conduit provided an implantation site for the valve in most patients. There was some modification of the device and delivery system after early problems however, the current design is frozen and is applicable for almost all clinical scenarios where a percutaneous valve is suitable for treating RVOT dysfunction. Furthermore, research is ongoing to expand the indications to include the morphological substrate not currently treatable by the present device.
Methods and results
Patients were considered for PPVI if they had previously undergone surgery on the right ventricular outflow tract (RVOT) as a part of repairing congenital heart disease, but had residual or recurrent RVOT dysfunction of sufficient degree to warrant further surgical intervention. This was defined as a right ventricular pressure > 2/3 of systemic pressure with evidence of outflow tract obstruction or significant pulmonary insufficiency leading to Right Ventricular (RV) dilatation with or without symptoms from RV failure.
We excluded patients < 5 years of age or weighing < 20 kg. Other exclusion criteria were pregnancy, occluded femoral veins, clinical signs of infection and outflow tracts with “unfavourable” morphology, defined as narrowest RVOT diameter > 22 mm or conduits < 16 mm in diameter.
Following a detailed history and physical examination, patients were assigned to a New York Heart Association (NYHA) functional class. Patients then underwent chest radiography, echocardiography (including pulsed and continuous wave Doppler) and whenever possible, Magnetic Resonance Imaging (MRI). Cardiopulmonary exercise testing for the analysis of respiratory gas exchange was performed to offer an objective assessment of functional status before and after PPVI. Student’s paired t test was used to evaluate the difference after intervention, and a 2-tailed probability value of P<0.05 was considered significant.
Of the 83 patients, 56% were male, median age 18 years and median weight of 56 kg. Tetralogy of Fallot and its variants formed the largest morphological substrate (n=48) followed by transposition of the great arteries (n=11) and truncus arteriosus (n=9). The rest included patients with pulmonary stenosis, post-repair of anomalous origin of the left coronary artery from pulmonary artery and after Ross operation.
After PPVI, the right ventricular systolic pressures dropped from 65±14 to 49±14 mm Hg (p< 0.001) and the pulmonary artery diastolic pressure increased from 10±3.6 to 13.4±7.6 (p<0.001). The right ventricular end-diastolic pressure decreased from a mean of 11.6 to 10.6 (p<0.05).
Magnetic resonance imaging demonstrated a significant drop in the pulmonary regurgitant fraction from 24±12% to 4±3% indicating the effectiveness of the valve in alleviating regurgitation in addition to reducing right ventricular hypertension.
The RV end-diastolic volume (EDV) decreased from 94±28 to 82±24 mL · beat–1 · m–2, (P<0.001) and there was a significant increase in RV effective stroke volume (37±7 to 42±9 mL · beat–1 · m–2, P=0.006) after PPVI. RVEF did not change (53±14% to 55±13%) (P=0.36).
There was a significant increase in LV EDV (64±12 to 71±13 mL · beat–1 · m–2, P=0.005) and LVSV (38±7 to 42±9 mL · beat–1 · m–2, P=0.005) without any change on the left ventricular ejection fraction.
There was a significant subjective improvement in NYHA functional class. Thirty-seven patients were in class III and IV with only seven patients in class I before PPVI. Most patients were in class I and II after PPVI (76/83) (Figure 1).
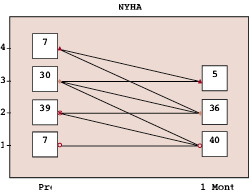
Figure 1. Pulmonary regurgitation fraction by magnetic resonance imaging after PPVI and at 1 year follow-up
This was also reflected on objective assessment by cardiopulmonary testing in a subset of these patients. There was a significant improvement in V O2 max (24.4±1.5 to 26.3±1.6 mL · kg–1 · min–1, P=0.009) and anaerobic threshold (13.5±1 to 15±0.9 mL · kg–1 · min–1, P<0.05).
There were some complications – one homograft rupture, 2 device dislodgements, and 1 coronary compression – however, most of these are learning curve related. These procedural complications led us to change our approach in similar situations and have not been encountered in the latter part of the series. Device related complications led to re-stenosis either by the “hammock effect” or stent fractures. The suturing of the valve to the stent was changed to eliminate the hammock effect successfully.
There were late explantations, some of which were device related. There are other factors that are not unavoidable, such as outgrown conduits in growing patients and stent fractures related to metal fatigue.
Discussion
Revision of RVOT is a common re-operation in patients with adult congenital heart disease. Stenosis of RV to PA conduits was successfully treated until now with balloon dilatation and bare metal stenting however, treatment of regurgitation was primarily surgical with replacement of the conduits. PPVI offers a safe and effective alternative for palliation of RVOT dysfunction, with elimination or reduction of gradient without increasing regurgitation.
When we compared our results to surgery during a contemporaneous period3 there was no mortality with PPVI (0/83) and low mortality with surgery (1/94). Major morbidities were seen in 8.5% (8/94) compared to 2.4% (2/83). Freedom from explantation for valve stenosis or regurgitation was over 90% at 1 year with the current design (Figure 2).
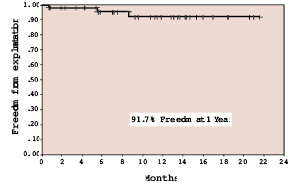
Figure 2. Freedom from explantation for valve stenosis or regurgitation by Kaplan-Meier method for the current valve design.
The success rate of implantation was very high due to the versatility of the delivery system (NuMED Inc.) which provides excellent trackability for a front-loaded device.
The cohort in this study were heterogeneous in terms of diagnosis, RV dilatation, and RV function and it is difficult to analyse the effect of this technique. Although the early results are encouraging, we hope the long term results show a sustained improvement. The risk of long term attrition of biological valves remains.
In summary, percutaneous pulmonary valve implantation continues to show early promise with a high success of implantation, and when the indications are well chosen, the results are excellent in the short and long term.

