Introduction
Mitral regurgitation (MR) is associated with an increase in mortality in patients with left ventricular dysfunction1, and more recently has also been associated with increased cardiac events even in asymptomatic patients2. The combination of a desire to improve survival as well as quality of life in symptomatic subjects accounts for the approximately 100,000 mitral valve operations performed worldwide annually. About half of these operations are mitral valve replacements, which can provide excellent reduction in mitral regurgitation and can be performed more quickly and by surgeons with less training than is necessary for mitral valve repair surgery. However, bioprostheses have limited longevity and mechanical valves require lifetime anticoagulation, which may limit their benefit. The potential for mitral valve repair to better maintain ventricular function and thereby improve patient survival has led to a recent increase in repair operations when the anatomy is suitable3.
Despite the reported advantages of mitral valve repair, no randomized trial has been conducted to compare repair with valve replacement. Observational studies that report the benefit of mitral valve repair often utilize the endpoint of freedom from re-operation rather than survival and report actual repairs as opposed to intention to treat. The latest generation of bioprostheses have demonstrated a marked improvement in valve longevity, and the use of chordal sparing may further improve the results of replacement. Both types of surgery are associated with significant morbidity in addition to mortality, which may be as high as 20%. In one study of Medicare patients undergoing mitral valve replacement, readmission within 30 days of surgery occurred in over 20% of patients4.
Repair procedures usually involve annuloplasty, and for degenerative valve disease frequently employ leaflet resection. In 1990, Alfieri introduced a novel surgical edge-to-edge repair technique involving the approximation of the middle scallops of the anterior and posterior mitral leaflets5. The results of this approach have been reported both in patients with and without ring annuloplasty with excellent results, even without a ring in patients with a noncalcified annulus6,7. Recently, a percutaneous procedure to create the same type of double orifice mitral valve was developed, and the results of a phase 1 safety and feasibility trial of this technique (EVEREST I) have been reported9. In this review, we will describe this procedure and report the additional results of the first 47 patients treated.
Methods
The procedure involves the use of a tri-axial catheter system (Evalve Incorporated, Menlo Park, California). The 24 French guide catheter, which tapers to 22 French in its distal portion, is inserted into the left atrium via a standard transseptal approach from the right femoral vein. The clip delivery system with the MitraClip attached at its distal end is then passed through the guide catheter into the left atrium. Both the guide catheter and the clip delivery system are steered by knobs on their proximal ends. The guide catheter can be flexed at the distal tip and the clip delivery system has two knobs to allow medial-lateral and anterior-posterior positioning. The clip itself is a polyester-covered mechanical device that can be opened and closed repeatedly to grasp the leaflets. The leaflets are secured by elements of the clip that grip and secure the atrial side of each leaflet. Once capture of the leaflets and creation of a double orifice mitral valve has been achieved, the clip is closed, locked, and released if MR reduction is adequate (Figure 1).
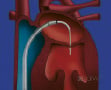
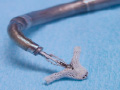

Figure 1. Upper left shows a drawing of the guide sheath inserted transseptally with the clip delivery system positioning the MitraClip just below the mitral valve in the left ventricle. Upper right panel shows the MitraClip in its open position with the grippers visible against the shaft. This is the clip position just prior to a leaflet grasp. Lower panel shows the proximal ends of the guide catheter and clip delivery system with the positioning knobs and clip detachment mechanism.
The clip can be repositioned on the leaflets as needed, or a second clip can be inserted if necessary to further reduce the degree of MR.
The procedure is performed under general anesthesia using primarily transoesophageal echocardiographic guidance. Echocardiography and a combined team approach is critical to the success of this procedure. The patients initially must be screened echocardiographically, not only for MR severity, but also for valve morphology. The severity of valve deformation can affect the ability to both grasp the leaflets and reduce MR effectively. Specific procedural echocardiographic views include: the basal short-axis for transseptal puncture, the mid-oesophageal, inter-commissural view for medial and lateral positioning and the mid-oesophageal long axis or left ventricular outflow tract view for anterior-posterior positioning and leaflet grasping. A pre-procedure strategy conference in order to review specific patient images and identify the most essential views to utilize during the procedure is helpful. Finally, it is important that the interventional cardiologist and echocardiographer utilize the same nomenclature in referring to the valve and patient anatomy10. Representative echocardiographic images from a patient treated with two clips are shown in Figure 2.
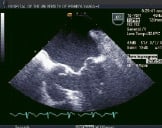
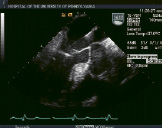
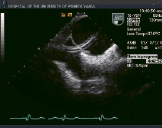
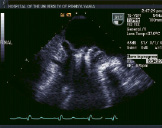
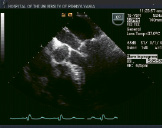
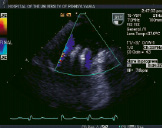
Figure 2. Top row from left to right shows TEE images of a patient treated with percutaneous edge-to-edge repair using the Evalve system. The first panel shows posterior leaflet prolapse into the left atrium. The middle panel is a short axis view at the base which demonstrated passage of the guide catheter across the inter-atrial septum. The right panel shows the MitraClip in its open position in the left atrium above the leaflets in a mid-oesophageal left ventricular outflow tract view. Bottom row from left to right: In the left panel, the MitraClip is positioned through the valve just prior to grasping the leaflets. The middle and right panels show two clips deployed on the mitral leaflets to create a double orifice with 2 colour flow jets of mitral inflow visible (right).
Results
EVEREST I (Endovascular Valve Edge-to-edge REpair Study) is a prospective, multicentre feasibility study with a primary safety endpoint for significant adverse events at 30 days. A secondary composite efficacy endpoint of MR severity is also collected and analysed by an echocardiographic core laboratory which uses the American Society of Echocardiography guidelines to quantify MR. The mean age of the first 47 patients treated in Everest I is 67 years11. Other clinical features of the study population include atrial fibrillation (40%), a history of congestive heart failure (51%), and a New York Heart Association classification of III or IV (48%). The etiology of mitral regurgitation is degenerative (87%) in most patients, and 13% of subjects had functional MR. A double orifice mitral valve was created during the procedure in all patients and clips were implanted in 42 patients, including 11 patients who received two clips. Clips were not implanted in five patients, most of whom were enrolled in the early experience when the use of two clips was not an option and insufficient MR reduction occurred. Seventy-four percent of this initial series of patients were discharged with the clip and MR less than or equal to 2+ as assessed by the echocardiographic core laboratory. The primary endpoint of freedom from 30-day significant adverse events was achieved in 96% of patients. Significant adverse events occurred in only two patients, one of whom required transfusion for bleeding from the femoral vein, and one of whom had a small, non-embolic stroke that resolved by 30 days12.
In midterm followup, a total of 12 patients have undergone surgery, including the five patients who did not receive a clip. Later operations (range 1 to 133 days) were performed in four patients due to a partial clip detachment from one leaflet without clip embolization, in two patients for ongoing mitral regurgitation, and in 1 patient due to a delivery catheter malfunction. Operations were valve repair in 9 patients and intended valve replacement in 3 patients. Importantly, there have been no partial clip detachments after 30 days, and in all patients requiring surgery, mitral valve repair or intended valve replacement was performed13. Mitral valve stenosis, a potential consequence of valve repair, was not detected at 12 months in any patient14.
Among 22 patients who were discharged with a clip and have undergone core laboratory echocardiographic follow-up evaluation at six months, 14 patients had 1+ or 2+ MR at one month and 13 of these 14 patients maintained this improvement at 6 months (8 of whom had 1+ MR) (Figure 3).
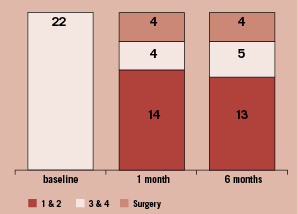
Figure 3. The number of patients who received clips and had core echocardiographic laboratory determined mitral regurgitation at baseline, 1 month, and 6 months are shown. Fourteen patients had 1+ or 2+ MR at one month and 13 of these 14 patients maintained this improvement at 6 months (8 of whom had 1+ MR).
Discussion
Based on these results, percutaneous edge-to-edge mitral valve repair with the Evalve MitraClip System is feasible and can successfully reduce and maintain mitral regurgitation to less than or equal to 2+ in the majority of patients. Although there is a steep learning curve for the initial performance of this procedure, experience and the option to use a second clip in some patients have reduced procedure time and increased efficacy. The rate of adverse events and complications was surprisingly low, and underlies the careful and controlled nature of the procedure despite its performance on a beating heart. Finally, it appears that in patients who do obtain a good result at one month, reduction in mitral regurgitation is maintained in midterm followup. Importantly, in patients who do require surgery in midterm followup, the use or attempt to use a clip did not appear to preclude the usual surgical options.
Echocardiography is an important and inherent tool to the successful performance of this procedure. A close collaboration between the interventional cardiologist and an “invasive” echocardiographer is essential to appropriately select patients, guide the procedure, and to assess the results. Collaborative decisions are made during the procedure to position the clip, confirm leaflet grasp and leaflet insertion, assess MR, and to decide on the potential need for a second clip. The echocardiographer must understand the technical aspects of the procedure and the interventional cardiologist needs to acquire a working understanding of mitral valve anatomy and transoesophageal echocardiographic views.
Based on the promising results in phase I, a phase II comparison with longer-term followup is now under way (Everest II). This trial is a prospective, randomised, multicentre comparison of surgical repair or replacement with percutaneous edge-to-edge repair using the MitraClip device. Patients with both degenerative and functional mitral regurgitation will be included, and all echocardiograms are graded for the degree of MR before and after treatment by a central core laboratory. One important contribution of this trial will be to provide the first prospective, core-laboratory monitored evaluation of the results of surgery for MR. The primary effectiveness endpoint for patients treated with a clip is the freedom from surgery for valve dysfunction, death, or 3-4+ mitral regurgitation at one year. Additional follow-up of patients in phase I as well as the comparative results with surgery in phase II will clarify the longterm durability and efficacy of this new technique.

