Introduction
Transcatheter repair of the mitral valve has entered the clinical stage based on surgical principles1,2. However, very few transcatheter techniques have been described for replacement of atrioventricular (AV) valves. In the experimental setting, valve implantation in the pulmonary and aortic position had been studied, leading us to try the same principle in the AV valve as well. We realized, in particular with our pulmonary project, that it was relatively easy to implant the prosthesis into the homograft rather than into the native tissue.
The implications are different for mitral valve due to problems of access, intimate relationship with the aortic valve and coronary circulation. Issues common to both AV valves are related to the size of the annulus at baseline, and more importantly in chronic regurgitation. The basic design was based on using the bovine jugular venous valve which was anchored to a nitinol stent frame which would fit the native annulus without causing any para-valvular leak.
Our approach was different for the tricuspid valve with implantation attempted within the native valve, however, in the mitral position, we attempted to implant a valve inside a bioprosthesis placed surgically3,4.
Methods and results
The device was different for tricuspid valve as the implant required a firm anchoring mechanism without leaving any paravalvular leak. A self expandable nitinol wire stent was developed formed by two separate discs separated by a tubular portion (AMF, Reuilly, France). The anchoring was by means of trapping the annulus between the two discs. The outer diameter was similar in dimension to the natural annulus diameter in ewes and the inner diameter matched the valve-diameter, which in this case was the stent- mounted bovine jugular venous valve. The nitinol stent was covered with a PTFE membrane to prevent any paravalvular leaks (Figure 1).
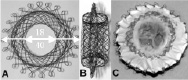
Figure 1. Design of the self expanding 0.22mm nitinol wire stent covered with PTFE.
For the mitral position, a porcine valve (Mosaic, Medtronic Inc.) was first implanted surgically, followed by off-pump implantation of valves over a delivery system introduced through the left atrium.
The delivery system consisted of a “home-made” front-loading 18-F long sheath (Cook Inc., Charenton le Pont, France). For the purpose of the study the distal tip of a dilator was cut off. The sheath could freely slide over the device. No balloon was necessary to deliver the nitinol stent that spontaneously deployed at the time of uncovering.
For the tricuspid valve, 10 ewes were divided into 2 groups based on when the animals were sacrificed. The first group was an acute study, group 2 ended at 1 month. The approach was through jugular or femoral veins and we did both haemodynamic and angiographic studies before and after the valve implantation. We monitored this through an epicardial echo through a left thoracotomy.
In group one, 4 of the 5 devices were successfully implanted with good function of the valves. In one animal it was impossible to completely deploy the valve despite attempts to dilate the device with a balloon catheter. In this animal, the device was not aligned with the tricuspid annulus. Angiographic and echocardiographic evaluations showed a severe paravalvular leak. At autopsy, the ventricular disk was trapped in the tricuspid chordae, explaining its incomplete deployment.
In group two, all devices were successfully implanted. There was no early or late stent migration. Evaluations showed that implants were in the desired position and confirmed the sealing of the device, showing no significant leak except in one animal, a significant paravalvular leak was found at the one-month evaluation. At autopsy, a weld fracture was seen in the stent, with a tear in the PTFE adjacent to it. Elsewhere, valves were competent and no other stent fractures were found. At autopsy, valve leaflets were thin and mobile in all animals. The valve was firmly embedded with endothelialization and it was not possible to remove without structural damage.
For the mitral valve, six animals had a 21 mm Mosaic aortic valve sheltering a 18 mm porcine valve as the first step followed by a trans-atrial implantation of a valved-stent without cardiopulmonary bypass. The valved-stent was a shortened CP stent with a bovine jugular venous valve sutured inside it. The delivery system was delivered over a wire placed into the left ventricle through an atrial puncture. The valve was deployed by sequential balloon inflations. Due to the small sized valves available, the end-point of this study was to judge successful delivery, appropriate position, alignment and absence of paravalvular leak. Valve implantation was successful in 5 out of 6 sheep. There was no paravalvular leak and aortic valve function was not affected. This was confirmed at autopsy.
Discussion
The clinical application of tricuspid valve replacement is currently quite limited. Tricuspid valve repairs for acquired tricuspid regurgitation would probably form the substrate for this technique rather than congenital anomalies such as Ebstein’s. However, this provides an alternative technique for intervention on regurgitant tricuspid valves.
The mitral valve is more complex due to its close proximity to the left ventricular outflow tract, coronary sinus, systemic operating pressures and transseptal approach. The device we used is a venous valve and has been shown to be operational at systemic pressures. Furthermore, the length of the implanted valve is limited by the length of the prosthetic substrate in our technique. Implantation within the native mitral annulus would be more difficult to design due to anatomical considerations. Also, the current design has a large profile which precludes percutaneous use from a peripheral vessel and further modification of the delivery system and the device. As transcatheter repair techniques are selective in modifications of the valve, we believe that valve implantation may become a way of dealing with most pathologies leading to atrioventricular valve regurgitation.

