Abstract
Aims: To report our experience and strategies with transcatheter closure of residual patent ductus arteriousus (RPDA) in patients with previous surgical ligation.
Methods and results: Transcatheter closure of residual patent ductus arteriousus after surgical ligation was attempted in 19 patients. In 13 patients the residual patent ductus arteriosus was closed with detachable coils, in four with Rashkind umbrella and in two with Amplatzer occluder. In order to cross the recanalised duct with the delivery system a vascular loop was required in six patients. Complete closure of residual ducts were achieved in all but one patient.
Conclusion: Transcatheter closure appears to be a safe and effective treatment for residual persistent duct. Coil implantation seems to be the best option in the case of smaller ducts, and in larger ones the Amplatzer Duct Occluder appears to be superior. Taking a meticulous approach to choosing the correct device should prevent ineffective treatment.
Introduction
Except for small children, percutaneous transcatheter closure has become the treatment of choice for patients with patent ductus arteriosus (PDA), however surgical closure is still performed in selected patients including premature infants. Residual patency may persist due to recanalisation or in the effect of incomplete closure of patent arterial duct, irrespective of previously undertaken procedure – surgical closure or interventional catheterisation1-4. The association between infective endarteritis and PDAs has been known for some time5. Some studies suggest that patients with residual PDAs may be at a greater risk for developing such complications6,7. Because of this we feel that it is necessary to identify these patients and complete the closure of the PDA. Precise data regarding percutaneous closure of residual PDAs are for the moment rare in the available literature8-10. The purpose of this study is to report on our experience and strategies with transcatheter closure of residual PDAs in patients with previous surgical ligation.
Patients and methods
Between October 1993 and September 2007 there were 287 patients operated due to persistent ductus arteriosus in our institution. In the same period nineteen of these patients were found to have residual post-surgical PDAs during follow-up (this group consisted also of patients from other institutions). In this group, the duct was double ligated but not divided. Clinical data of these patients are presented in Table 1.
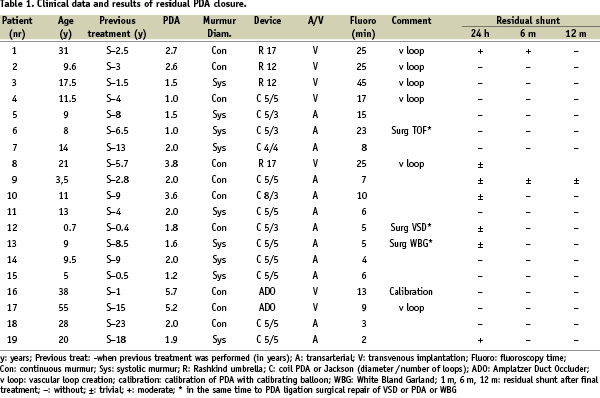
A continuous murmur was found in nine patients. In all the remaining patients systolic murmur was present. Three patients had additional cardiovascular problems and were operated previously for: tetralogy of Fallot (patient six), ventricular septal defect (patient 12), and Bland-White-Garland syndrome (patient 13). Only one patient in our series (case one) had symptoms of congestive heart failure (class III of the New York Heart Association). Residual shunts were diagnosed in clinical examination and ultrasound Doppler, then confirmed by angiography before a reintervention was scheduled. Diameter measurements of the residual PDAs were performed by aortography in all but one patient (number 16) whose ductal measurement was performed with the aid of a calibration balloon catheter (AGA Medical Corporation, Golden Valley, MN, USA). Reinterventions were performed from 1.0 – 23 (mean 7.1) years after surgical ligation.
In the majority of patients, residual PDA occlusion was performed with detachable coils. A PDA double disc occluder (USCI Rashkind Umbrella, size 12 or 17 millimetre) was applied for residual PDA closure in four patients (in the years 1993 - 96). In two patients with a very wide PDA, 5.7 and 5.2 millimetre diameter (patient numbers 16 and 17), Amplatzer Duct Occluders (ADO – 10/8 millimetre) were applied (AGA Medical Corporation, Golden Valley, MN, USA) (Figure 1).
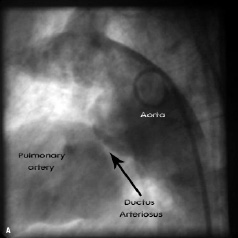
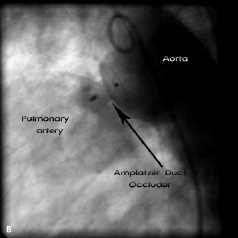
Figure 1. Aortography (lateral projection). 1a: Residual shunt after surgical ligation of the patent arterial duct; 1b: Percutaneous closure by the Amplatzer Duct Occluder
Detachable coils (manufactured by William Cook Europe, Bjaeverskov, Denmark) were implanted according to the morphology of the PDA: eight 5PDA5, three 5PDA3, one 8PDA3 and one Jackson MWCE 38-4-4. In seven patients, the PDA was crossed antegrade and in all others retrograde. Direct cannulation appeared impossible in six patients. In order to pass through the PDA with the delivery system a vascular loop was created (veno-arterial in three patients and arterio-venous in three). Implantation of the devices was performed according to reported protocols11-13. All patients underwent periodic follow-up in our outpatient clinic by ultrasound Doppler sampling with codified colour (Hewlett-Packard Medical, now acquired by Philips Medical System, Andover, MA, USA). Only one patient was lost to follow-up (Case 8 - Table 1).
The median follow up was 6.3 years (from 0.4 to 12.1).
Results
Percutaneous closure was attempted in all 19 patients (Table 1). There were no deaths or significant morbidity (arterial or venous puncture complications, haemolysis etc.). Obstruction of the left pulmonary artery and the descending aorta was excluded echocardiographically in all patients. The PDA diameter ranged from 1.0 to 5.7 (mean 2.25).
A total number of 19 devices were successfully implanted in 19 patients without any embolisation during or after the procedure. The complete closure of patent arterial duct was achieved within 12/19 (63%) patients 24 hours after percutaneous treatment, and 16/17 (94%) after one year. In patient #1, who had a clinically significant shunt despite two unsuccessful surgical ligation attempts, a moderate residual shunt was present six months after the implantation of a 17 millimetre Rashkind umbrella. The shunt completely disappeared after one year of follow-up. Fluoroscopy time ranged from two to 45 (mean 13.3) minutes. Generally, the procedures in which the necessity of vascular loop creation was presented took a longer time. There were no complications related to the procedures. All patients were discharged home the next day after clinical and ultrasound Doppler examination. Until now, there have been no cases of residual PDA after transcatheter treatment. No patient had developed infective endarteritis during follow-up.
Discussion
In the majority of patients with patent arterial duct, except the smallest infants, percutaneous closure has replaced surgery as the choice of treatment. Regardless of the method, the goal of therapy is to achieve complete and persistent closure with a single procedure. However, at times physicians are still faced with the problem of residual leaks. There are many issues surrounding the closure of residual PDAs that still remain unclear. For example, there are no clearly agreed guidelines when deciding whether a residual PDA should be closed or simply monitored. We believe that treatment of a PDA is the appropriate approach in order to prevent complications from endarteritis and lifelong antibiotic prophylaxis. In our institution, as in many others, we have had success in treating residual PDAs percutaneously, yet specific data in the literature on interventional closure of residual PDAs are rare9,10. In this same period, the incidence of residual leaks after percutaneous interventions was 3.6 % (14 patients from a group numbering a total of 418 patients) in our institution. In this study we have reported on our experience with 19 cases of residual PDAs after surgical ligation.
Due to the relatively large number of residual shunts after the Rashkind Umbrella implantation we searched for other devices9,12-16. In our study, we analysed the period from 1993 till 2007. During this time we have seen clear progress in transcatheter therapy with the development of coils and the Amplatzer Duct Occluder. Currently, detachable coils are our preferred devices for percutaneous closure of a smaller residual PDAs17. In cases of larger residual PDA’s, our device of choice is the Amplatzer Duct Occluder. In a few circumstances, with larger residual PDAs, it is possible to use one larger coil or simultaneously apply two coils. Although it is an option, we do not recommend this because the risk of embolisation or persistence of residual shunt seems to be higher. Failure of total closure of a medium sized PDA could be secondary to the use of coils instead of the Amplatzer Duct Occluder. To avoid this, we definitively changed our policy applying to the Amplatzer Duct Occluder in wider PDAs (even 2-2.5 mm in diameter). On the other hand, according to our strategy, even small silent PDAs found on aortography should be closed percutaneously, if it is possible to cross the PDA. In that manner, we believe, we can prevent infective endarteritis. In these smaller ducts the coil seems to be the best therapeutical option.
Previously it had been argued that surgical treatment of the patent arterial duct was superior to the transcatheter techniques, and it was believed that only a few ducts remained patent after surgical closure18,19. However, when patients who had surgical ligation of the duct underwent the assessment with colour Doppler flow mapping, residual flow was found in 6-23% patients1,2. Currently the residual shunting after PDA surgery is becoming a rarity. We believe it is the consequence of the improvement of surgical methods, like standard double or triple ligation and division of the PDA. Because the majority of our post-surgical patients were directed to us from other hospitals, it was very difficult to define the real number of residual PDAs after surgical ligation in our cohort. Inadequate surgical techniques undertaken during patent arterial duct operations were the most possible reason for the larger residual leaks (all patients were double ligated but not divided). This data may suggest that residual PDAs following surgical closure may indeed present with larger duct diameters and possibly more clinically significant PDAs.
Transcatheter closure of residual PDAs may be a difficult procedure at times due to changes in the ductal anatomy. These changes are often caused by tissue adhesions, torsion or kinking provoked by sutures. Special techniques may be required to cannulate or cross the residual duct with the delivery catheter. In such circumstances, a vascular loop creation can be beneficial, as in patients one, two, three and eight (where Rashkind umbrellas were implanted), in patient four (with coils applied), and in patient 17 (with ADO implantation). A different, and helpful solution, is to implant a coil in a wedge position without passing the catheter into the pulmonary artery.
In conclusion, according to our experience, we can say that transcatheter closure of residual PDAs is a safe and effective treatment. We believe that it is important to identify and treat residual PDAs in order to prevent potential long term complications and lifelong antibiotic prophylaxis, although more studies should be done to help define indications for reintervention. For smaller residual PDAs, the detachable coil implantation seems to be the best option in view of it’s relatively low cost, safety profile, and effectiveness. In our opinion the Amplatzer Duct Occluders are especially useful in the interventional treatment of larger recanalised ducts. In situations where transcatheter closure is complicated by altered ductal anatomy, certain techniques, including vascular loops and wedged coil implantations, can be used to successfully close the residual PDA. By choosing adequate devices it is possible to avoid ineffective treatments.

