Introduction
A steady rise in the prevalence of chronic cardiovascular diseases coupled with new insights into tissue healing has stimulated an interest in the potential of progenitor cell-mediated repair and regeneration. The administration of biologic agents via catheters has a long history and, in recent years, research and development teams have turned their attention to creating devices specifically for delivering cell products. Catheters used for cell delivery are varied in fundamental ways, and do not fall into a “one-size fits all” group. As evaluation of these devices continues1, a clearer picture is emerging as to the capabilities and limitations of percutaneous cell delivery.
The goals of cell-based therapeutics are to promote tissue repair and regeneration. As depicted in Figure 1, the methods now used involve a series of steps, two of which (cell processing and delivery) themselves are multi-staged. Irrespective of the cell type or preparation being tested, or whether its desired effects occur through the release of intracellular mediators and/or the maturation and integration (engraftment) into specific tissue, maximising the retention of cells after administration is paramount to the procedure’s success. Unfortunately, the ability of the heart to retain an injection of cells is poor and the literature is replete with discouraging data. Of the total administered dose of cells (or other similar material), no more than 35-40% are detectable at one hour2 and 10% at one day3. Moreover, rates of retention appear similar and independent of type of cell, delivery technique or disease state4, although variability by injection method exists for specific agents5. The mechanisms of cell attrition are very different in the environment of high interstitial flow (normal myocardium) versus that of ischaemic and fibrotic tissue. While individual steps in a specific method of cell administration may influence cell retention, all are dependent to a large degree on the effectiveness of the delivery technique.
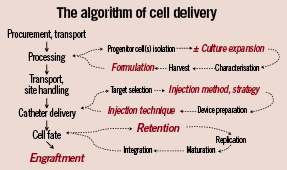
Figure 1. Algorithm of cell delivery. The flow diagram of a typical, stepwise process for procuring and administering cell products. Highlighted in red are those parts of the process which that may have a larger role in affecting cell retention and engraftment.
Despite significant strides made during the past 2 years, the field of percutaneous cell delivery is at an early stage of development. To date, it has not developed measures of procedure success, depriving itself of a parameter valuable to intra- and inter-study comparisons. Nevertheless, even in its present form, it holds clear advantages over alternative delivery strategies: 1) higher efficiency of cell delivery compared peripheral intravenous injection, 2) lower procedure risk-profile compared to surgery, 3) high potential for integration into clinical practice, in that many techniques are modifications of current interventional methods, and 4) higher facility for repeat applications compared to surgery, which is still the benchmark for intramyocardial injections. Regarding the last point, it is assumed that catheter-based delivery will provide a level of efficiency equal to (or greater than) surgery, although data comparing the techniques are scarce, especially in diseased myocardium. From several studies the comparison appears to be favourable6-9. Hou et al9, observed insignificant differences in the retention of radio-labelled mononuclear cells given by intramyocardial (surgical), intracoronary or retrograde venous approaches in a one week old porcine-MI model. Nevertheless, no study dedicated to the rigorous assessment of cell injections into advanced myocardial disease with these techniques has been reported; nor have clinical trials attempted to do so as yet.
In this paper, we present an overview of percutaneous cell delivery techniques and devices, focusing on those devices in clinical or late-stage preclinical study. In doing so, we will present as comprehensive a picture as possible, by highlighting the features of individual devices, by identifying the issues they face and by discussing potential solutions to enhancing the effectiveness of these techniques.
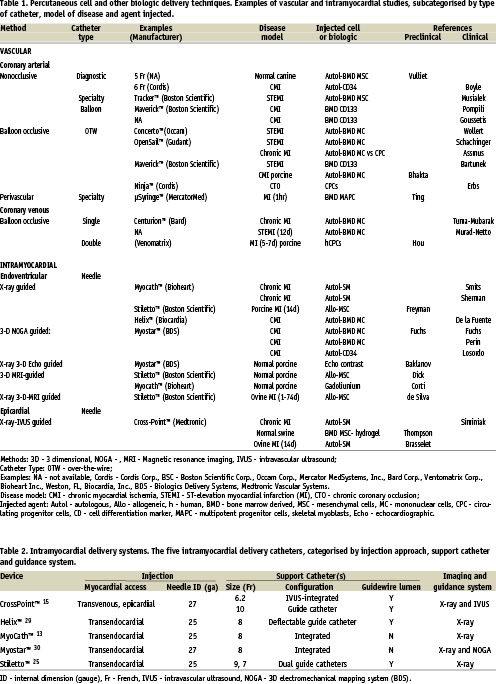
Techniques and devices (Table 1)
The techniques for percutaneous cell injection fall broadly into 2 categories: “coronary vascular” or “intramyocardial”. For the most part, the primary goal for these techniques is to provide access of injected cells to the microvasculature or extravascular space (interstitium), or both.
Coronary vascular techniques require that target tissue be served by angiographically identifiable vessels (even if only collateral channels), and that the desired effect(s) of the cell product occur through any combination of: 1) endothelial adhesion, 2) transvascular migration, 3) physical interruption of the endothelial barrier through hydrostatic or mechanical force, 4) cytokine release, 5) maturation and integration into diseased tissue (engraftment). The most commonly applied coronary vascular methods are antegrade arterial. In particular, sub-selective cell injections through the central lumen of an over-the-wire (OTW) angioplasty catheter, while either maintaining coronary flow or interrupting it with balloon occlusion (the so-called, “stop-flow” method). This technique is simple, utilises off-the-shelf devices and has been the method of choice for nearly all studies in patients with STEMI10-16 in addition to other clinical conditions17. Diagnostic, guide and specialty catheters are suitable for nonselective cell infusion, given the calibre of their internal diameters (ID), although there is limited experience in their off-label use at this time18. Surprisingly, the kinetics of coronary arterial cell delivery have received little attention in preclinical studies19. Unlike the lead-in to the first clinical trials of IC gene products, the transition of cell-based studies from small animals20 to humans10 was rapid. Consequently, much of what is known regarding cell retention after IC administration has been learned from clinical studies3,21.
The other category of vascular administration is retrograde venous. A solid body of data underlies this approach9,22-24, and, in part, builds upon clinical experience in the administration of non-cellular agents. Directing cell products to the post-capillary vasculature circumvents several of the technical problems of arterial methods, especially those related to occlusive arterial disease. Theoretically, all vascular territories are accessible, although the full geometric breadth of this technique has not been reported.
Intramyocardial methods administer cell products by the insertion of small calibre needles directly into the ventricular wall. By necessity, devices capable of this are comprised of 2 or more components and are more aptly described as “catheter systems”, especially in that several are coupled to dedicated imaging modalities. The two essential elements are the injection (core) lumen for biological delivery and the support catheter that enables directional positioning of needle tip. Other elements specific to individual devices permit redirection of the support catheter, transmission of image data, infusion/irrigation of lumina in addition to the core and passage of guidewires.
The intramyocardial catheter systems that have seen use in clinical trials are outlined in Table 2. The needle size (25-27ga) is comparable to all. Otherwise, they differ by virtue of access to the myocardium (transendocardial or transepicardial), of mechanisms of navigation (integrated “tip-deflectable” vs steerable guide vs transvenous-IVUS-directed) and of ventricular imaging. The integrated design provides a relatively simple mechanism for intra-cavitary navigation and repeated injections. However, without a guidewire lumen their passage from femoral artery to left ventricular chamber is dependent on those same navigation mechanisms or the use of long sheaths to facilitate access. Other differences between the five devices relate to needle composition (stainless steel or nitinol), configuration (straight, curved or helical) and activation (manual or spring loaded), and methods of catheter imaging.
All intramyocardial catheter systems in Table 2 use X-ray imaging for either a part or all of the procedure. Adjunctive imaging for catheter guidance is incorporated into the two systems. One (Myostar™25) utilises electromechanical sensing of the endocardium and is particularly well suited to delineating ischaemic and non-ischaemic tissue. The other (CrossPoint™), uses IVUS imaging to orient the needle on its initial trajectory from the coronary venous system into the myocardium. Passage of the injection catheter through the needle is then visualised by fluoroscopy. Other modalities have also been used, including integrated real-time MRI2,26,27, 3D-echocardiography28 and, more recently, a combination NOGA-imaging and automated catheter positioning system (Stereotaxis) has been piloted (E. Perin, P. Serruys, personal communication). MRI and 3D echo systems permit the visualisation of intramyocardial injections, although only the latter is readily available.
The functional differences among or between vascular and intramyocardial devices have not been fully explored, although comparative data is emerging29.
The issues: a 3-component analysis
As can be surmised from Figure 1, problems within this complex algorithm can arise at any step, including during cell injection. Methods of cell processing and handling, variability in biologic effects of individual cell preparations (especially autologous products), the lack of simple potency assays and other issues affect all cell delivery methods. The factors bearing on the immediate results of catheter delivery largely arise from interactions of the 3 components of the delivery process: cell product, cardiac tissue, delivery technique, and the magnitude of effect for each will vary variable, depending on the Table 3 lists the cell preparations in current use, or soon to be used in human studies. Clinical trials have been completed with bone marrow-derived mononuclear cells, mesenchymal stem cells and immunoselected populations (CD 34+, CD 133+, mesenchymal precursor). Multipotent adult progenitor cells (MAPCs), adipose-derived and cardiac-derived cells are in late-stage preclinical evaluations. In general, cell products are tested for viability (% cells, by methylene blue exclusion), purity (% cells of specific cell-type) and sterility (by gram stain) prior to administration; it is upon these three characteristics that “release criteria” are based and that determine a product’s suitability for experimental use. Less often assayed is the “functionality” or “potency” of a cell preparation, i.e., those in vitro properties (maturation, migration, myotube formation), that may predict in vivo behaviour.
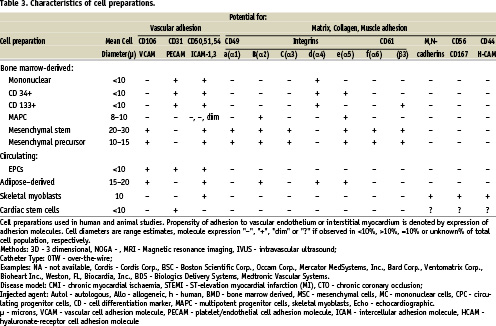
From the procedural perspective, an important feature of a cell product is its response to alterations in environment, such as passage into a syringe or through a delivery catheter, or release into the in vivo milieu. Such characteristics contribute to the early fate of cells after injection, and can be inferred from their in vitro expression of adhesion molecules. A framework for predicting cell behaviour upon initial contact with recipient tissue is presented in Table 3, in which cell size and surface molecule expression are denoted. The propensity for attachment to vascular- extravascular (interstitial matrix, collagen) bound ligands is appreciated from this table and is information that needs to be factored into the choice of the delivery method. Since cell products are often comprised of mixed populations, the presence of specific adhesion molecules is best defined as a% of cells. However, we have used >10% cell-expression as positive in Table 3, and cell diameter as a range. Variability is therefore inherent to this schema and it cannot account for changes in expression that may occur after implantation.
Nevertheless, the profiles of expressed adhesion molecules suggest that both bone marrow and adipose-derived cells will readily attach to vascular endothelium, less reliably to extracellular matrix tissues and with little affinity for myocardium. The muscle progenitors (skeletal myoblasts and cardiac-derived stem cells) express the most surface markers for adhesion to the intersitium and/or collagen, although mesenchymal-type cells demonstrate this capacity as well.
Not included in the Table 3 are those factors that promote tissue growth, such as vasculogenesis or myogenesis, or those that facilitate migration. When such characteristics are combined with adhesion molecule expression, a more thorough profile of a cell product is created. From this, insight can be gained into its interaction with delivery catheters and recipient tissue, and a better estimation of cell retention.
Issues specific to catheter delivery are listed in Table 4 and grouped according to interactions of three major components: 1) the cell preparation; 2) the delivery system; and 3) the recipient tissue. A separate category identifies those issues that fall within the operator’s direct control. Each component brings specific effects to the interaction and, unfortunately, most interactions negatively influence cell retention. Even though we have made no attempt to quantify the relative bearing of each component on cell retention, the nature of the underlying disease and the characteristics of the cell product are, in fact, primary, and will often precede the choice of delivery system in formulating a study design.
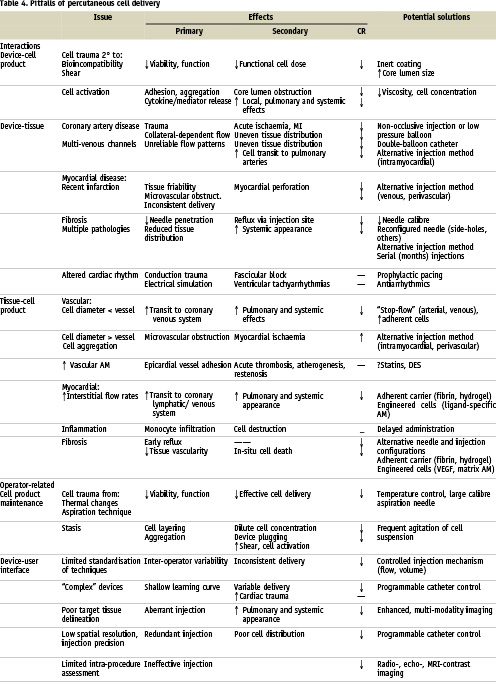
Interactions between delivery device and cell preparation may result in cell trauma (from shear effects or bio-incompatibility) and impairment of viability and function. The release of cytokines and other mediators may be hastened, leading to their early appearance locally and systemically. Cells may adhere to the device’s lining or form aggregates, narrowing the core lumen diameter and changing the flow characteristics during delivery. Fortunately, most device-cell interactions are easily identified in bench-top testing, allowing for adjustments to be made in either component prior to in vivo use.
Although methods for percutaneous catheter delivery have thus far been generally safe, risks from adverse device-tissue interactions exist. Coronary artery and myocardial trauma are the most worrisome30,31; disruption of normal cardiac rhythm30 are largely temporary. Impairment of cell retention can result from unfavourable interrelationships between devices and tissues. In vascular delivery methods, obstructive coronary artery disease with inaccessible collateral channels or coronary venous anatomy with multiple communications both lead to shunting of cell injections away from target tissues32. Intramyocardial injection methods are plagued by myocardial fibrosis and tissue inhomogeneity, which can lead to inadequate needle penetration33.
Tissue-cell interactions. Ideally, injected cells will be retained in numbers sufficient to optimise their biologic effects. With coronary vascular delivery, the steps of adhesion and transvascular migration add to the challenge of cell retention. These processes involve paracrine signalling, and various adhesion molecules including integrins, Ig superfamily cell adhesion molecules (CAMs) and selectins. Among the highest rates of cell retention and engraftment comes with the cost of myocardial ischaemia and infarction29. By combining an understanding of surface marker expression and cell dimension, an acceptable balance between microvascular adhesion and obstruction can be achieved, especially with regard to arterial delivery. On a larger scale, interactions of cells with epicardial vessels may have acute and chronic implications34,35. The interactions between myocardial tissue and cell product also relate to surface marker profile and the underlying disease state, perhaps more so with the latter. The high interstitial flow rates of normal myocardium36 lead to rapid clearance of most agents injected intramyocardially. A very different situation exists in chronic fibrosis, in which the loss of cells is related more to leakage through the injection track and to local ischaemia.
The operator controls the key aspects of percutaneous cell delivery. Maintenance of the cell product, by protecting it from injury (thermal or mechanical) or stasis prior to loading into the delivery system, is a simple and important task. By preventing cell layering, the chances of administering doses containing mostly media or cell aggregates are reduced. The latter, as noted above, may promote unfavourable device-cell interactions.
More positively challenging to the operator are the complex aspects of novel delivery systems and their imaging modalities. Intramyocardial injection catheters function within a different framework than the vascular devices and, while a fair body of experience exists with some30,37-39, most have seen limited use in clinical trials to date. Even though none are overly demanding in concept or mechanics, the learning curves are still being charted for all of them, especially in states of advanced myocardial disease.
Excluded from Table 4 are two issues of particular importance to device and biotechnology companies: product adaptability and strategic partnering. Both are crucial for hypothesis testing and for discerning optimal pathways to product approval. As is now evident, delivery methods for cell-based tissue repair become increasingly complex when combined with the many variables of clinical diseases and cell characteristics. The design and conduct of preclinical and clinical studies often leave little room for error, and recovery from early misdirection can be challenging.
Summary
With its established successes in catheter-based therapies, the interventional community might expect the delivery of progenitor cells to the heart to be a straightforward task, since the coronaries, even if occluded, offer access to essentially all cardiac tissue and should therefore be effective conduits for cellular products. However, it has become clear that progenitor cell survival is poor after injection, irrespective of the sophistication of the delivery catheter or the expertise of the operator. Among the issues facing percutaneous delivery, including those pertaining to safety, cell retention is the foremost.
Together with underlying pathology and characteristics of specific cell preparations, percutaneous delivery techniques form the three major components of cell-based tissue repair. Interplay between them is variable and dependent on their unique properties: the nature (and stage) of disease, the immediate (and delayed) behaviour of the cell product, the ability of the catheter to effectively deliver cells with minimal trauma to cells or tissue. An understanding of the potential interactions is necessary in developing a study design. Unfortunately, since most interactions between the three components appear to reduce cell retention, the most we can expect is to minimise the losses.
Presently, there is no “preferred” technique for cell delivery, but, rather, a continued need to categorise existing and newer systems by their physical characteristics and capabilities. Multiple pathologies, tissue inhomogeneity and diversity of cell products have given rise to delivery methods that are more complex than their predecessors, or when compared to routine coronary interventions. The selection of a delivery method should be preceded by an assessment of the other two components in the decision matrix, rather than serving as the starting point. As the field evolves and as experience is gained, we will be better prepared to define a “successful injection” and the importance of integrating optimal delivery methods into pivotal studies. Then it will be possible to evaluate cell preparations and to interpret clinical trials results on the basis of the success, or failure, of the percutaneous catheter to deliver the study agent.

