Aims
The non-anatomical properties of conventional bench-top testing apparatus hinder the pre-clinical testing of percutaneous heart valve technology. The aim of this work was to construct a system that integrates a mechanically re-animated post-mortem heart with an artificial circulation and multi-modality imaging to further enable the study of percutaneous heart valve therapies.
Methods and results
Model
A fresh postmortem swine heart (300g) was excised from the animal’s chest and cannulated with an 18 mm clear polyvinyl tube in the ascending aorta. The inferior pulmonary vein was incised to allow the insertion of a 30 mm clear PVC medical grade tube. A 12.5 mm fluid-tight trocar was positioned in the superior pulmonary vein (Thoracoport™, Norwalk, CT). All tubes and trocars were secured in the tissue using 0-silk suture and the chain technique. A 12.5 mm fluid-tight trocar with a side-port for a pressure transducer was positioned in the apex of the left ventricle using a pledgeted 0-silk pulse string secured with a tourniquet.
The tube in the ascending aorta of the post-mortem heart was attached to the plastic ascending aorta of a replica of the human peripheral circulation (Angiogram Sam™, MPL, Gateville,TX). The ends of the plastic vessels in the replica were sealed with silicone plugs, with the exception of the vessels of the plastic aortic arch and the left and right plastic femoral artery’s in which Touhy-Borst access ports were attached (Qosina, Edgewood, NY). The left carotid of the plastic vessel was attached to a length of 5/8 inch medical grade tubing in which the distal end of the tube was suspended 3.3 meters above the heart. The fluid exiting the tube was collected by a funnel and fed into a volumetric collecting flask. A six foot long section of 5/8 inch clear medical grade tubing was connected to the tube in the inferior pulmonary vein and fed passively with water from an open-air adjustable-height 10L reservoir above the level of the heart to provide water at 15 mmHg pressure into the left atrium. A pressure transducer was attached by a stopcock to this each set of tubing at the level of the heart (Millar Mikro-tip Catheter, Millar Instruments, Houston, TX).
Ultrasound examination
A Transoesophageal echocardiography probe was introduced inside a latex tube orientated in the anatomical position of the excised oesophagus on the posterior surface of the postmortem heart (latex tube-Aircomp Ultralight™ Michelin, Paris, France). The latex tube and the posterior surface of the heart were submerged in a water bath.
“Animation” of the postmortem heart
A docking station for the heart was designed and constructed to allow circumferential nylon bands to be adjustably orientated in respect to the heart (Figures 1A and B).
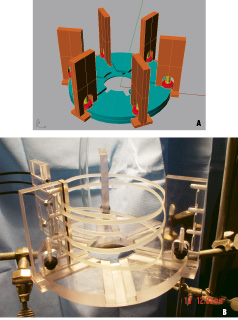
Figure 1A. Schematic of postmortem heart docking platform. Figure 1B. Actual docking platform with multiple nylon bands aligned to constrict heart in center (heart not shown). Black cables (upper left) attach to motor mount.
The end of the band was attached to a coated wire cable which was connected to a computer-controlled motor mount (Figures 2A and B).
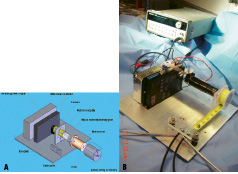
Figure 2A. Schematic of motor mount, exploded view. Figure 2B. Actual motor mount. Black cables (lower center) connect to docking platform. Motor mount controlled by programmable microprocessor in background.
The computer was programmed to cyclically pull the band a variable distance (1-4 inches) at a programmable number of pulls per minute (0-300 pulls/minute).
Construction of the percutaneous valve
A trileaflet heart valve was constructed from glutaraldehyde-fixed bovine pericardium (Edwards LifeSciences Inc, Irvine, CA) using a method previously described1. The valve was mounted on a 20 mm diameter self-expandable stainless steel braided stent using 7-0 sutures at three points, 120 degrees apart.
Delivery and deployment
The valve was compressed in a 20F delivery catheter and inserted into Touhy-Borst trocar in the tube representing the right femoral artery of the plastic model. The catheter was advanced in a retrograde manner across the native aortic valve under direct visualization from the endoscope. The retrograde procedure was repeated for the second valved stent which was prematurely deployed before it reached the nadirs of the inferior aspect of the aortic annulus.
Visualization of the valve
During animation of the heart, the endoscopes allowed simultaneous superior and inferior views of the opening and closing of the aortic and mitral valves (Figure 3).

Figure 3. Simultaneous endoscopic visualization of aortic and mitral valves from an endoscope in ascending aorta (upper view) and second endoscope in apex of post-mortem heart (lower view).
Millar catheter pullback across the native aortic valve (Figures 4A-C) measured the transvalvular pressure changes generated by the imposed band pulling cycles.
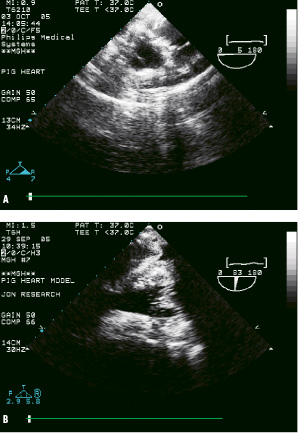
Figure 4A. Short-axis ultrasound view of native aortic valve in “re-animated” post-mortem heart, and, 4B. Long-axis ultrasound view of the mitral valve from artificial oesophagus.
Ultrasound images of the native aortic and mitral valve leaflets were visible from the artificial oesophagus (Figures 5A-B).
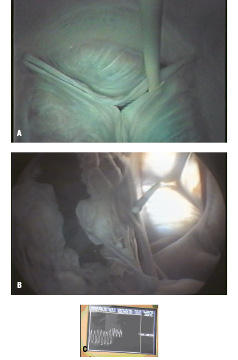
Figure 5A. Millar catheter pullback across native aortic valve, superior endoscopic view. 5B. Same catheter, inferior endoscopic view. Mitral valve open (left) and aortic valve closed (right). 5C. Transvalvular pressure gradient tracing from millar pullback across native aortic valve.
The endoscopic views provided visualization of the left coronary ostia and the anterior leaflet of mitral valve throughout the deployment and seating of the percutaneous valve replacement (Figures 6A-D).
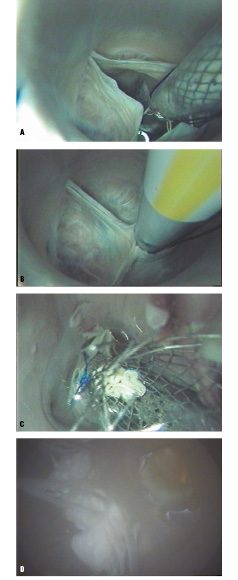
Figure 6A. Delivery of self-expanding valved stent across aortic valve, superior endoscopic view. 6B. Aortic leaflets in closed position around delivery catheter. Note: left coronary ostia in sinus, lower left. 6C. Deployed percutaneous valve replacement fully expanded in aortic position. 6D. View of same aortic valve replacement from inferior endoscope. Note: anterior leaflet of mitral valve, left of seated aortic valve.
Para-valvular space
The prematurely deployed valve was seated approximately 1 cm superior to the nadir of the base of the aortic annulus. A para-valvular space was visible from the inferior endoscopic view, which revealed the exact orientation and dimensions of the space with respect to the neighboring anatomy and the device (Figure 7).
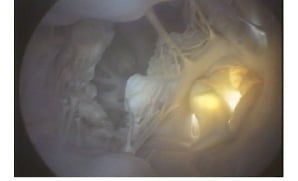
Figure 7. Inferior endoscopic view of mal-positioned percutaneous aortic valve with transilluminated paravalvular space at 7 o’clock position of seated aortic valve on right.
Conclusion
Serious complications including death were reported in the first group of patients undergoing percutaneous aortic valve replacement therapy leading to the abrupt halt of the clinical trials of this technology2. Device malpositioning, migration during deployment, damage to neighbouring anatomical structures, and para-valvular leakage were major contributing factors in the complications experienced by this group3. Pre-clinical testing of these devices was hampered by unsuitable bench-top test systems and poor animal models.
Conventional bench-top heart valve testing apparatus use models that lack important anatomical features4. In addition, they fail to represent variations in the anatomical relationships of important structures, such as the sinuses of Valsalva or coronary ostia. In the system described in this study, the integration of a post-mortem animal heart into a bench-top apparatus enabled the endoscopic visualization of the valved stent during its delivery and subsequent deployment. Simultaneous visualization of anatomic structures and the device enabled detection of displacement or obstruction of adjacent structures.
One of the potentially most valuable aspects of an adaptable biologic apparatus is the ability to accommodate postmortem human hearts and animal hearts. Important differences between synthetic and biologic systems can be modelled, including biomechanical properties, implant site distensibility and the resultant effects of cyclic loading.
The conventional apparatus used in the pre-clinical testing of valves is constructed from synthetic materials which inaccurately models the biomechanics of the body’s tissue. Valves are secured in these apparatuses by suturing or mechanically clamping the valve to the synthetic implant site. Since percutaneous valves are secured to the implant site by expansion of the stent, the standard systems are limited in their ability to test percutaneous valves for malpositioning during deployment or migration under physiologic pressure changes. In contrast, biologic systems allow for various fluid pressures to be exerted on the valved stent after it is deployed into tissue having the anatomical configuration of the intended implant site in patients.
The ability of the expanded valved stent to oppose the cyclic load of fluid forces and maintain a stable position at the implant site can be viewed from multiple views by the endoscopes. Although this system does not provide chronic data of valve stability, it would be useful for detecting and studying acute device migration, particularly those caused by malpositioning or inappropriate sizing of the valved stent to the implant site. Techniques for human implant often rely on rapid pacing of the heart (220 beats/min). Since the rate of the heart re-animation can be programmable, it allows modelling of this effect on the placement of the valve during the retrograde delivery.
Other specifics of device placement in humans are difficult to model in animals. The peripheral vessels in ovine, bovine, or canine models are too small in diameter to insert the guiding catheters for the valved stents5. This necessitated investigators to use more central access routes to test the deployment devices with much different approach trajectories. Such approaches may mask the detection of potential complications during deployment.
In the first trial of percutaneous aortic valve replacement trans-septally, deaths occurred due to severe mitral regurgitation caused by laceration of the anterior leaflet of the mitral valve from the deployment guidewire6, a finding not reported in any of the published pre-clinical animal studies, highlighting the limitation of the models used. The visualization of the anterior leaflet of the mitral valve from the inferior endoscope in this system may allow for early detection of designs which result in critical impingement of the guidewire on the leaflet during antegrade deployment of a valve.
Preclinical investigation of percutaneous valve technology in animal models was hampered by device migration7. Early investigators largely dismissed the relevance of these findings to humans, since these complications were thought to be attributable to the anatomy of animals or the lack of native valve pathology such as stenosis or calcification. Unfortunately, however, a patient in the first group undergoing percutaneous aortic valve replacement experienced device migration with catastrophic results8. The use of postmortem human hearts combined with an artificial circulation in the apparatus as described in this paper should help prevent the migration of various devices by facilitating the studying of their function in an anatomical setting under physiologic conditions, prior to their use in patients.
In clinical trials of the first generation of percutaneous aortic valve replacements, para-valvular leakage was frequent and often a limiting factor in device success9. Aortic para-valvular leak in patients with chronic aortic stenosis is poorly tolerated by the hypertrophied left ventricle, an important clinical sequelae, not modelled in the acute animals used for standard pre-clinical valve testing. The pre-clinical animal studies provided overly optimistic findings regarding para-valvular leakage. In these studies, the degree of para-valvular leakage was minimal or absent, most likely due to the lack of native valve pathology which causes physical irregularity at the valve implant site in humans. In this study, the malpositioned valve demonstrated a para-valvular space, endoscopically visualized in the inferior view, enabling detailed examination of the para-valvular space in relationship to the device and neighboring anatomy. By providing accurate information of the para-valvular space, this system would facilitate the testing of valves designed to prevent para-valvular leakage.
Multi-modality imaging of anatomy, including endoscopic images of the heart are largely unavailable in the clinical setting. When these views are correlated with ultrasonic and angiographic imaging, it offers the potential for physician training, as well as testing of new devices and procedures using multi-modality imaging in the pre-clinical setting.

